-
Name
Boc-Glycine
- EINECS 224-864-5
- CAS No. 4530-20-5
- Article Data113
- CAS DataBase
- Density 1.159 g/cm3
- Solubility Soluble in water
- Melting Point 86-89 °C(lit.)
- Formula C7H13NO4
- Boiling Point 315.9 °C at 760 mmHg
- Molecular Weight 175.185
- Flash Point 144.9 °C
- Transport Information UN 2811 6.1/PG 3
- Appearance white to off-white powder
- Safety 26-36/37/39-39-36
- Risk Codes 22-41-36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms Boc-Gly-OH;tert-Butoxycarbonylglycine;2-(tert-butoxycarbonylamino)acetate;Nalpha-tert-Butyloxycarbonylglycine;t-Butoxycarbonylglycine;N-(Carbo-tert-butoxy)glycine;Glycine, N-[ (1, 1-dimethylethoxy)carbonyl]-;tert-Butyloxycarbonylglycine;N-t-Butyloxycarbonyl glycine;N-[(1, 1-Dimethylethoxy)carbonyl]glycine;N(a)-tert-Butyloxycarbonylglycine;2-(tert-butoxycarbonylamino)acetic acid;N-(tert-butoxycarbonyl)glycine;Glycine, N-carboxy-, N-tert-butyl ester;Glycine, N-carboxy-, N-tert-butyl ester (8CI);N-((1,1-Dimethylethoxy)carbonyl)glycine;N-tert-Butyloxycarbonylglycine;N-t-BOC-Glycine;[(tert-butoxycarbonyl)amino]acetic acid;N-Boc-glycine;
- PSA 75.63000
- LogP 0.98660
Synthetic route

| Conditions | Yield |
|---|---|
| With potassium hydroxide In 1,4-dioxane; water at 20℃; for 12h; | 100% |
| Stage #1: glycine With sodium hydroxide In 1,4-dioxane; water at 0℃; for 0.25h; Stage #2: di-tert-butyl dicarbonate In 1,4-dioxane; water at 0 - 20℃; | 100% |
| With sodium hydroxide In 1,4-dioxane | 100% |
-
-
83316-95-4
2-oxo-2-phenylethyl 2-(tert-butoxycarbonylamino)acetate

-
A
-
4530-20-5
BOC-glycine

-
B
-
98-86-2
acetophenone

| Conditions | Yield |
|---|---|
| With N,N,N',N'-tetramethyl-o-phenylenediamine In acetonitrile for 2h; Irradiation; | A 100% B n/a |

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal for 3h; | A 100% B n/a |

-
A
-
4530-20-5
BOC-glycine

-
B
-
1033736-87-6
C16H10O3S

| Conditions | Yield |
|---|---|
| In chloroform-d1 for 0.25h; Solvent; Photolysis; Inert atmosphere; | A 97% B n/a |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 1,4-dioxane at 20℃; for 8h; | 95% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; acetone at 25℃; for 12h; | 94% |
-
-
64205-15-8
(N-hydroxy-5-norbornene-2,3-diformylimino)tert-butyl ester

-
-
56-40-6
glycine

-
-
4530-20-5
BOC-glycine

| Conditions | Yield |
|---|---|
| With tertiary amine In 1,4-dioxane; water for 3h; | 85% |
-
-
909114-65-4
tert-butyl 4,6-dimethoxy-1,3,5-triazinyl carbonate

-
-
56-40-6
glycine

-
-
4530-20-5
BOC-glycine

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; for 0.5h; | 85% |
-
-
79113-14-7
4-nitrobenzyl N-(tert-butoxycarbonyl)glycinate

-
-
4530-20-5
BOC-glycine

| Conditions | Yield |
|---|---|
| With sodium dithionite; sodium carbonate In acetonitrile | 80% |

| Conditions | Yield |
|---|---|
| With triethylamine In 1,4-dioxane; water for 0.166667h; Ambient temperature; | 80% |
-
-
83316-95-4
2-oxo-2-phenylethyl 2-(tert-butoxycarbonylamino)acetate

-
A
-
4530-20-5
BOC-glycine

-
B
-
6926-09-6
(tert-butoxycarbonyl)glycine hydrazide

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In methanol Product distribution; | A 75% B 12% |
-
-
618068-89-6
N-Boc-glycine 2-(2-acetylphenyl)-1-methylethyl ester

-
-
4530-20-5
BOC-glycine

| Conditions | Yield |
|---|---|
| In acetonitrile for 3h; Photolysis; | 73% |
-
-
1080004-01-8
(E)-3-methoxyallyl 2-((tert-butoxycarbonyl)amino)acetate

-
-
4530-20-5
BOC-glycine

| Conditions | Yield |
|---|---|
| With zinc(II) chloride; lithium hexamethyldisilazane In tetrahydrofuran at -78 - 20℃; Inert atmosphere; | 59% |
| With potassium hexamethylsilazane; zinc(II) chloride In tetrahydrofuran at -78 - 20℃; Inert atmosphere; | 54% |
-
-
42721-06-2
tert-Butoxycarbonylamino-acetic acid (2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-hydroxymethyl-tetrahydro-pyran-2-yl ester

-
-
5680-79-5
glycine ethyl ester hydrochloride

-
A
-
4530-20-5
BOC-glycine

-
B
-
53487-98-2
N-tert-butoxycarbonyl glycyl glycine methyl ester

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine In N,N-dimethyl-formamide at 38℃; for 96h; | A n/a B 57% |

-
-
5680-79-5
glycine ethyl ester hydrochloride

-
-
35909-78-5
2,3,4,6-tetra-O-benzyl-1-O-(N-tert-butoxycarbonyl-glycyl)-β-D-glucopyranose

-
A
-
4530-20-5
BOC-glycine

-
B
-
53487-98-2
N-tert-butoxycarbonyl glycyl glycine methyl ester

-
-
4132-28-9, 4291-69-4, 6386-24-9, 6564-72-3, 59531-24-7, 69257-52-9, 78184-89-1, 78609-16-2, 78609-17-3, 78609-18-4, 96553-53-6, 104111-61-7, 131347-08-5
2,3,4,6-tetra-O-benzyl-D-glucopyranose

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine In N,N-dimethyl-formamide at 38℃; for 96h; | A n/a B 55% C n/a |


| Conditions | Yield |
|---|---|
| With sodium carbonate In water; acetonitrile at -5 - 20℃; | 35% |
-
-
6456-74-2
Glycine tert-butyl ester

-
-
83316-95-4
2-oxo-2-phenylethyl 2-(tert-butoxycarbonylamino)acetate

-
A
-
4530-20-5
BOC-glycine

-
B
-
5845-68-1
Boc-glycylglycine tert-butyl ester

| Conditions | Yield |
|---|---|
| In ethyl acetate for 24h; Product distribution; Ambient temperature; | A 10% B 30% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide; tert-butyl alcohol |

| Conditions | Yield |
|---|---|
| With toluene Erwaermen des Reaktionsprodukts mit tert-Butylalkohol und anschliessenden Hydrolyse; |

| Conditions | Yield |
|---|---|
| With triethylamine Behandeln des Reaktionsprodukts mit wss.NaOH und Dioxan; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| Stage #1: BOC-glycine; benzylamine With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide for 0.0166667h; Stage #2: With HATU for 18h; | 100% |
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; | 99% |
| With zirconium(IV) chloride In tetrahydrofuran at 70℃; for 24h; Molecular sieve; Inert atmosphere; | 99% |
-
-
4530-20-5
BOC-glycine

-
-
27894-50-4
methyl (2S)-2-amino-6-{[(benzyloxy)carbonyl]amino}hexanoate hydrochloride

-
-
32689-62-6
Nα-tert-butoxycarbonylglycyl-Nε-benzyloxycarbonyl-L-lysine methyl ester

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane at -20 - 20℃; for 4h; Acylation; | 100% |
| Stage #1: BOC-glycine With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane for 0.5h; Cooling with ice; Stage #2: methyl (2S)-2-amino-6-{[(benzyloxy)carbonyl]amino}hexanoate hydrochloride With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; | 92.2% |
| (i) Et3N, ClCO2iBu, (ii) /BRN= 3578476/, Et3N; Multistep reaction; | |
| With 4-methyl-morpholine; triethylamine; isobutyl chloroformate 1) THF, -10 deg C, 15 min, 2) THF, 12 h; Yield given. Multistep reaction; |
-
-
4530-20-5
BOC-glycine

-
-
42854-62-6
L-alanine benzyl ester p-toluenesulfonate

-
-
63649-08-1
Boc-Gly-Ala-OBzl

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine | 100% |
| With TEA; diphenyl phosphoryl azide In N,N-dimethyl-formamide 1.) 0 deg C, 4 h; 2.) r.t., overnight; | 85% |
| Stage #1: BOC-glycine; L-alanine benzyl ester p-toluenesulfonate With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane for 0.166667h; Inert atmosphere; Stage #2: With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 24h; Inert atmosphere; |
-
-
4530-20-5
BOC-glycine

-
-
16652-71-4
benzyl (2S)-2-pyrrolidinecarboxylate hydrochloride

-
-
29776-78-1
(S)-1-(2-tert-butoxycarbonylaminoacetyl)pyrrolidine-2-carboxylic acid benzyl ester

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; triethylamine In chloroform | 100% |
| With benzotriazol-1-ol; triethylamine In chloroform | 100% |
| With benzotriazol-1-ol; triethylamine In chloroform | 100% |
-
-
4530-20-5
BOC-glycine

-
-
16652-76-9
L-valine benzyl ester p-toluenesulfonate salt

-
-
66415-00-7
N-t-butoxycarbonylglycyl-L-valine benzyl ester

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine | 100% |
| With 4-methyl-morpholine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In tetrahydrofuran at 0 - 20℃; for 12h; Inert atmosphere; | 94% |
| With benzotriazol-1-ol; triethylamine; dicyclohexyl-carbodiimide In N,N-dimethyl-formamide 1.) 0 deg C, 2.) overnight, 4 deg C; | 89% |
| With 4-methyl-morpholine; isobutyl chloroformate 1.) THF, -15 deg C, 2.) DMF, a) -15 deg C, 30 min, b) r.t., 30 min.; Yield given. Multistep reaction; |
-
-
4530-20-5
BOC-glycine

-
-
65253-04-5
(-)-8-phenylmenthol

-
-
117681-86-4
(1R,2S,5R)-2-(1-methyl-1-phenylethyl)-5-methylcyclohexyl (tert-butoxycarbonyl)aminoacetate

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 13h; | 100% |
| Stage #1: BOC-glycine With dmap; dicyclohexyl-carbodiimide In dichloromethane at -30℃; for 0.166667h; Stage #2: (-)-8-phenylmenthol In dichloromethane for 20h; Heating; | 96% |
| With dmap; dicyclohexyl-carbodiimide | 93% |
-
-
4530-20-5
BOC-glycine

-
-
26690-80-2
2-(N-tert-butoxycarbonylamino)ethanol

| Conditions | Yield |
|---|---|
| Stage #1: BOC-glycine With 4-methyl-morpholine; isobutyl chloroformate In tetrahydrofuran at -15℃; for 0.25h; Stage #2: With sodium tetrahydroborate In tetrahydrofuran; water at -15℃; for 1h; | 100% |
| With diisopropoxytitanium(III) tetrahydroborate In dichloromethane for 4h; Ambient temperature; | 88% |
| With sodium bis(2-methoxyethoxy)aluminium dihydride In tetrahydrofuran; toluene at 0℃; for 2h; | 77.1% |
-
-
4530-20-5
BOC-glycine

-
-
100-39-0
benzyl bromide

-
-
54244-69-8
tert-butoxycarbonylamino-acetic acid benzyl ester

| Conditions | Yield |
|---|---|
| Stage #1: BOC-glycine With caesium carbonate In methanol pH=7; Stage #2: benzyl bromide In N,N-dimethyl-formamide at 20℃; for 5h; Further stages.; | 100% |
| Stage #1: BOC-glycine With caesium carbonate In N,N-dimethyl-formamide at 20℃; for 0.25h; Stage #2: benzyl bromide In N,N-dimethyl-formamide at 0℃; for 18h; Further stages; | 100% |
| With triethylamine In N,N-dimethyl-formamide at 25℃; for 5h; | 54% |
| With caesium carbonate 1) MeOH, water, pH 7.0, 2) DMF, 5 h; Yield given. Multistep reaction; | |
| With sodium iodide In acetone for 2h; Reflux; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| Stage #1: BOC-glycine; ethyl iodide With sodium hydride In tetrahydrofuran at 0℃; for 1h; Stage #2: In tetrahydrofuran at 0℃; | 100% |
| Stage #1: BOC-glycine; ethyl iodide With sodium hydride In tetrahydrofuran at 0 - 20℃; Stage #2: With citric acid In water pH=2 - 3; | 100% |
| Stage #1: BOC-glycine; ethyl iodide With sodium hydride In tetrahydrofuran at 0 - 20℃; Stage #2: With citric acid In water pH=2 - 3; | 100% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In N,N-dimethyl-formamide at 50℃; for 16h; | 100% |
| With N-ethyl-N,N-diisopropylamine for 0.666667h; Reflux; | 91% |
| With caesium carbonate In acetonitrile | 90% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In tetrahydrofuran at 25℃; for 5h; | 100% |
| With fluorosulfonyl fluoride; N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 5h; | 99% |
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In tetrahydrofuran at 20℃; for 6h; | 96.9% |
-
-
4530-20-5
BOC-glycine

-
-
543-27-1
isobutyl chloroformate

-
-
66866-43-1
N-(t-butoxycarbonyl)glycyl i-butyl carbonate

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine In tetrahydrofuran at -15℃; for 0.0833333h; | 100% |
| With 4-methyl-morpholine In tetrahydrofuran at -20℃; for 0.05h; |
-
-
4530-20-5
BOC-glycine

-
-
52199-35-6
tetrakis-5,10,15,20-(o-aminophenyl)porphyrin

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at -5 - 20℃; for 50h; Condensation; | 100% |
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane Inert atmosphere; Cooling with ice; | 62% |
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane |

| Conditions | Yield |
|---|---|
| Stage #1: H-Ala-Glu(OBzl)-OBzl hydrochloride With triethylamine In dichloromethane Hydrolysis; Stage #2: BOC-glycine With benzotriazol-1-ol; dicyclohexyl-carbodiimide In dichloromethane at 0 - 25℃; for 14h; Condensation; | 100% |
-
-
4530-20-5
BOC-glycine

-
-
64-04-0
phenethylamine

-
-
613675-75-5
tert-butyl N-{[(2-phenylethyl)carbamoyl]methyl}carbamate

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; benzotriazol-1-ol; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In acetonitrile at 0 - 20℃; Acylation; | 100% |
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In ethyl acetate at 20℃; for 72h; | 94% |
| Stage #1: BOC-glycine With 1,1'-carbonyldiimidazole In dichloromethane at 20℃; for 1h; Stage #2: phenethylamine In dichloromethane for 16h; | 81% |
| Stage #1: BOC-glycine With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; triethylamine In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: phenethylamine In N,N-dimethyl-formamide at 20℃; | 72% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 23℃; for 4h; Inert atmosphere; | 71% |
-
-
33941-15-0
1-aza-18-crown-6

-
-
4530-20-5
BOC-glycine

-
-
394208-14-1
[2-oxo-2-(1,4,7,10,13-pentaoxa-16-aza-cyclooctadec-16-yl)-ethyl]-carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With bis(trichloromethyl) carbonate; lutidine In tetrahydrofuran at 25 - 50℃; for 2h; | 100% |
| With 2,6-dimethylpyridine; bis(trichloromethyl) carbonate In tetrahydrofuran Inert atmosphere; |
-
-
4530-20-5
BOC-glycine

-
-
172611-73-3
4-{N-[1-(4,4-dimethyl-2,6-dioxocyclohexylidene)-3-methylbutyl]amino}benzyl alcohol

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 24h; | 100% |
-
-
4530-20-5
BOC-glycine

-
-
191593-14-3
4,6-O-di(tert-butyl)silanediyl-D-glucal

-
-
848359-00-2
3-O-(N-tert-butoxycarbonyl-2'-amino-ethanoyl)-4,6-O-di-tert-butylsilanediyl-D-glucal

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane | 100% |
-
-
4530-20-5
BOC-glycine

-
-
2450-71-7
Propargylamine

-
-
847490-49-7
tert-butyl (2-oxo-2-(prop-2-yn-1-ylamino)ethyl)carbamate

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane for 6h; Inert atmosphere; | 100% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 16h; Inert atmosphere; | 99% |
| With benzotriazol-1-ol; triethylamine; dicyclohexyl-carbodiimide In acetonitrile at 20℃; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: BOC-glycine; anthranilic acid With pyridine; triphenyl phosphite at 150℃; for 0.166667h; microwave irradiation; Stage #2: 2-methoxyethylamine at 220℃; for 0.0666667h; microwave irradiation; | 100% |


| Conditions | Yield |
|---|---|
| Stage #1: BOC-glycine; anthranilic acid With pyridine; triphenyl phosphite at 150℃; for 0.166667h; microwave irradiation; Stage #2: 2-methoxyethylamine at 180℃; for 0.05h; microwave irradiation; | 100% |

-
-
916452-54-5
(3S,10R,16S)-16-{(1R)-3-[4-(hydroxymethyl)phenyl]-1-methylallyl}-3-isobutyl-10-(4-methoxybenzyl)-1-oxa-4,8,11-triazacyclohexadec-13-ene-2,5,9,12-tetraone

-
-
4530-20-5
BOC-glycine

-
-
916452-55-6
tert-butoxycarbonylamino-acetic acid 4-{3-[3-isobutyl-10-(4-methoxy-benzyl)-2,5,9,12-tetraoxo-1-oxa-4,8,11-triaza-cyclohexadec-13-en-16-yl]-but-1-enyl}-benzyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 3h; | 100% |
-
-
4530-20-5
BOC-glycine

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; HATU In dichloromethane for 3h; pH=9; | 100% |
-
-
4530-20-5
BOC-glycine

-
-
89226-13-1
2-(N-t-butoxycarbonylamino)thioacetamide

| Conditions | Yield |
|---|---|
| With Lawessons reagent In dichloromethane at 25℃; for 16h; | 100% |
| Stage #1: BOC-glycine With ammonium hydroxide; triethylamine; isobutyl chloroformate In tetrahydrofuran Stage #2: With tetraphosphorus decasulfide; sodium carbonate In tetrahydrofuran | 83% |
| Multi-step reaction with 3 steps 1: Et3N / tetrahydrofuran / 0.42 h / -10 °C 2: aq. MH3 / tetrahydrofuran / 0.75 h / -10 °C 3: 85 percent / Lawesson's reagent / CH2Cl2 / 16 h / Ambient temperature View Scheme |
-
-
4530-20-5
BOC-glycine

-
-
540-37-4
p-aminoiodobenzene

-
-
216774-64-0
[(4-iodophenylcarbamoyl)methyl]carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With dacarbazine; dmap In dichloromethane | 100% |
| With dmap; diisopropyl-carbodiimide In dichloromethane for 20h; Inert atmosphere; | 100% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane at 20℃; for 5h; | 98% |
-
-
4530-20-5
BOC-glycine

-
-
68641-49-6
bis-(2-oxo-3-oxazolidinyl)phosphoryl chloride

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane | 100% |

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 5h; | 100% |
Boc-glycine Chemical Properties
Molecular structure of Boc-glycine (CAS NO.4530-20-5) is:
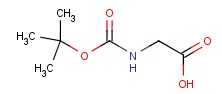
Product Name: Boc-glycine
CAS Registry Number: 4530-20-5
IUPAC Name: 2-[(2-methylpropan-2-yl)oxycarbonylamino]acetic acid
Molecular Weight: 175.18242 [g/mol]
Molecular Formula: C7H13NO4
XLogP3-AA: 0.5
H-Bond Donor: 2
H-Bond Acceptor: 4
EINECS: 224-864-5
Melting Point: 86-89 °C(lit.)
Water Solubility: soluble
Surface Tension: 40.1 dyne/cm
Density: 1.159 g/cm3
Flash Point: 144.9 °C
Enthalpy of Vaporization: 61.27 kJ/mol
Boiling Point: 315.9 °C at 760 mmHg
Vapour Pressure: 9.08E-05 mmHg at 25°C
Product Categories: Protected Amino Acids;Aminoacids Derivatives;Amino Acid Derivatives;Protected Amino Acid & Peptides;Amino Acids;Glycine [Gly, G];Boc-Amino Acids and Derivative;Amino Acids 13C, 2H, 15N;Absolute Configuration Determination (Exciton Chirality CD Method);Amino Acids (N-Protected);Analytical Chemistry;Biochemistry;Boc-Amino Acids;Enantiomer Excess & Absolute Configuration Determination;Exciton Chirality CD Methodfor (for Monofunctional Compounds);Boc-Amino acid series;Amino Acids & Derivatives
Boc-glycine Safety Profile
Safty information about Boc-glycine (CAS NO.4530-20-5) is:
Hazard Codes:  Xn,Xi
Xn,Xi
Risk Statements: 22-41-36/37/38-20/21/22
R22:Harmful if swallowed.
R41:Risk of serious damage to the eyes.
R36/37/38:Irritating to eyes, respiratory system and skin.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.
Safety Statements: 26-36/37/39-39-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S39:Wear eye / face protection.
S36:Wear suitable protective clothing.
RIDADR: UN 2811 6.1/PG 3
WGK Germany: 3
Boc-glycine Specification
Boc-glycine , its cas register number is 4530-20-5. It also can be called 2-[(2-Methylpropan-2-yl)oxycarbonylamino]acetic acid ; Glycine, N-[(1,1-dimethylethoxy)carbonyl]- ; N-(tert-Butoxycarbonyl)glycine ; Nalpha-tert-Butyloxycarbonylglycine ; t-Butoxycarbonylglycine ; Glycine, N-carboxy-, N-tert-butyl ester (8CI) .It is a white to off-white powder.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View