-
Name
dioctanoyl peroxide
- EINECS
- CAS No. 762-16-3
- Article Data19
- CAS DataBase
- Density 0.955g/cm3
- Solubility 7μg/L at 20℃
- Melting Point 29 °C
- Formula C16H30 O4
- Boiling Point 353.5°C at 760 mmHg
- Molecular Weight 286.412
- Flash Point 149.3°C
- Transport Information
- Appearance
- Safety A peroxide. Handle carefully. When heated to decomposition it emits acrid smoke and irritating vapors.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Octanoylperoxide (6CI,8CI); Caprylyl peroxide; Di-n-octanoyl peroxide; Dicaprylylperoxide; Dioctanoyl peroxide; Perkadox SE 8; Peroyl O
- PSA 52.60000
- LogP 4.70880
Caprolyl Peroxide Chemical Properties
Chemistry informtion about Caprolyl Peroxide (CAS NO.762-16-3) is:
IUPAC Name: Octanoyl Octaneperoxoate
Synonyms: Dioctanoyl Peroxide ; Caprylyl Peroxide. ; Dioctanoylperoxid ; Dicaprylyl Peroxide ; Octanoic Acid Octaneperoxoic Anhydride
MF: C16H30O4
MW: 286.407
EINECS: 212-094-2
Density: 0.955 g/cm3
Flash Point: 149.3 °C
Boiling Point: 353.5 °C at 760 mmHg
Vapour Pressure: 3.57E-05 mmHg at 25°C
Enthalpy of Vaporization: 59.84 kJ/mol
Following is the molecular structure of Caprolyl Peroxide (CAS NO.762-16-3) is:
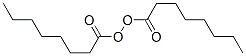
Caprolyl Peroxide Consensus Reports
Reported in EPA TSCA Inventory.
Caprolyl Peroxide Safety Profile
A peroxide. Handle carefully. When heated to decomposition it emits acrid smoke and irritating vapors.
RIDADR: 3106
HazardClass: 5.2
PackingGroup: II
Caprolyl Peroxide Specification
Caprolyl Peroxide (CAS NO.762-16-3) is a straw-colored liquid with sharp odor.It is highly flammable and water insoluble. Peroxides, such as dioctanoyl peroxide, are good oxidizing agents. Organic compounds can ignite on contact with concentrated peroxides. Strongly reduced material such as sulfides, nitrides, and hydrides may react explosively with peroxides. There are few chemical classes that do not at least produce heat when mixed with peroxides. Many produce explosions or generate gases (toxic and nontoxic). Generally, dilute solutions of peroxides (<70%) are safe, but the presence of a catalyst (often a transition metal such as cobalt, iron, manganese, nickel, or vanadium) as an impurity may even then cause rapid decomposition, a buildup of heat, and even an explosion. Solutions of peroxides often become explosive when evaporated to dryness or near-dryness. Danger of explosion when dry.
Related Products
- Caprolyl Peroxide
- 7621-86-5
- 762-21-0
- 762217-20-9
- 76-22-2
- 7622-23-3
- 7622-29-9
- 762239-61-2
- 762240-09-5
- 762240-92-6
- 762260-71-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View