-
Name
Choline chloride
- EINECS 200-655-4
- CAS No. 67-48-1
- Article Data39
- CAS DataBase
- Density 1.205 g/cm3
- Solubility soluble in water
- Melting Point 302-305 °C (dec.)(lit.)
- Formula C5H14ClNO
- Boiling Point
- Molecular Weight 139.625
- Flash Point
- Transport Information
- Appearance White crystalline powder
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Choline hydrochloride;Choline chloride solutions;(2-Hydroxyethyl)trimethylammonium chloride;Cholinum;Ethanaminium, 2-hydroxy-N,N, N-trimethyl-, chloride;Hormocline;Colina cloruro [DCIT];2-Hydroxy-N,N,N-trimethylethanaminium chloride;Ammonium, (2-hydroxyethyl)trimethyl-, chloride;2-Hydroxy-N,N,N-trimethylethanaminium;Cholinium chloride;sell choline chloride;Bilineurin chloride;Ethanaminium, 2-hydroxy-N,N,N-trimethyl-;Paresan;2-hydroxyethyl-trimethyl-azanium;Neocolina;Choline chlorhydrate;Lipotril;2-Hydroxy-N,N,N-trimethylammonium chloride;Choline, chloride;Chloride de choline [French];Chlorure de choline [INN-French];Biocolina;Cholini chloridum [INN-Latin];2-hydroxyethyl-trimethyl-azanium chloride;
- PSA 20.23000
- LogP -3.31110
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 24.9℃; Thermodynamic data; ΔrH1; | 100% |
| With sodium hydroxide at 24.9℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol; acetonitrile at 70℃; for 14h; Inert atmosphere; | 98% |
| at 80℃; | |
| With water |

| Conditions | Yield |
|---|---|
| With diethylamine at 40℃; for 9h; | A 88% B n/a |

-
-
64-17-5
ethanol

-
A
-
330957-88-5
ethyl 3-amino-1-(4-bromo phenyl)-1H-benzo[f]chromene-2-carboxylate

-
B
-
67-48-1
choline chloride

| Conditions | Yield |
|---|---|
| With diethylamine at 40℃; for 9h; | A 84% B n/a |

-
-
64-17-5
ethanol

-
A
-
299937-04-5
ethyl 3-amino-1-(3-nitrophenyl)-1H-benzo[f]chromene-2-carboxylate

-
B
-
67-48-1
choline chloride

| Conditions | Yield |
|---|---|
| With diethylamine at 40℃; for 10h; | A 83% B n/a |

-
-
64-17-5
ethanol

-
A
-
330957-87-4
ethyl 3-amino-1-(4-nitro phenyl)-1H-benzo[f]chromene-2-carboxylate

-
B
-
67-48-1
choline chloride

| Conditions | Yield |
|---|---|
| With diethylamine at 40℃; for 10h; | A 74% B n/a |

-
-
64-17-5
ethanol

-
A
-
67-48-1
choline chloride

-
B
-
119825-14-8
ethyl 3-amino-1-(4-methoxyphenyl)-1H-benzo[f]chromene-2-carboxylate

| Conditions | Yield |
|---|---|
| With diethylamine at 40℃; for 11h; | A n/a B 73% |

-
-
64-17-5
ethanol

-
A
-
67-48-1
choline chloride

-
B
-
130944-14-8
ethyl 3-amino-1-(4-chlorophenyl)-1H-benzo[f]chromene-2-carboxylate

| Conditions | Yield |
|---|---|
| With diethylamine at 40℃; for 10h; | A n/a B 70% |

-
-
64-17-5
ethanol

-
A
-
405109-32-2
ethyl 3-amino-1-(2-nitrophenyl)-1H-benzo[f]chromene-2-carboxylate

-
B
-
67-48-1
choline chloride

| Conditions | Yield |
|---|---|
| With diethylamine at 40℃; for 10h; | A 70% B n/a |


| Conditions | Yield |
|---|---|
| With diethylamine at 40℃; for 11h; | A 68% B n/a |

-
-
64-17-5
ethanol

-
-
1277100-30-7
C25H27N2O3(1+)*Cl(1-)

-
A
-
67-48-1
choline chloride

-
B
-
84186-26-5
2-amino-3-ethoxycarbonyl-4-phenyl-4H-naphtho<2,1-b>pyran

| Conditions | Yield |
|---|---|
| With diethylamine at 40℃; for 8h; | A n/a B 67% |


| Conditions | Yield |
|---|---|
| With diethylamine at 40℃; for 12h; | A 66% B n/a |

-
-
64-17-5
ethanol

-
A
-
67-48-1
choline chloride

-
B
-
130944-15-9
ethyl 3-amino-1-(4-hydroxy phenyl)-1H-benzo[f]chromene-2-carboxylate

| Conditions | Yield |
|---|---|
| With diethylamine at 40℃; for 12h; | A n/a B 60% |


| Conditions | Yield |
|---|---|
| With boron fluoride ether; acetone |

| Conditions | Yield |
|---|---|
| beim Umkrystallisieren; |

| Conditions | Yield |
|---|---|
| With silver nitrate |

| Conditions | Yield |
|---|---|
| With potassium hydroxide | |
| Stage #1: ethanolamine; methyl iodide With potassium carbonate for 12h; Stage #2: With silver(l) oxide In methanol for 0.25h; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; trimethylamine at -12 - -10℃; Erhitzen auf 80-90grad; |

| Conditions | Yield |
|---|---|
| With macrocyclic polyphenolate; water at 60℃; Rate constant; Equilibrium constant; Mechanism; pH 10.0; ionic strength 0.10 M; var. pH; |

| Conditions | Yield |
|---|---|
| With water at 30℃; for 0.166667h; Rate constant; relative rate, different alkaline phosphotases; |

| Conditions | Yield |
|---|---|
| With sodium phosphate buffer at 25.2℃; Rate constant; Mechanism; Electrophorus electricus acetylcholinesterase (EC 3.1.1.7), pH 7.26, μ=0.3 (0.06 N NaCl), also Torpedo californica acetylcholinesterase; other choline and thiocholine esters; |
-
-
81372-19-2
<2-(n-dodecylmethyl-tert-butylsiloxy)ethyl>trimethylammonium nitrate

-
A
-
67-48-1
choline chloride

-
B
-
81372-22-7
n-dodecylmethyl-tert-butylsilanol

| Conditions | Yield |
|---|---|
| With potassium fluoride In water-d2 at 25℃; for 24h; stability/lability; var. solvents, reagents and reaction times; |
-
-
7647-01-0
hydrogenchloride

-
-
816-94-4, 4539-70-2, 66701-63-1, 816-93-3
{2-[(2,3-bis-stearoyloxy-propoxy)-hydroxy-phosphoryloxy]-ethyl}-trimethyl-ammonium betaine

-
A
-
67-48-1
choline chloride


-
-
67-48-1
choline chloride

| Conditions | Yield |
|---|---|
| bei der Autolyse; |

-
-
67-48-1
choline chloride

| Conditions | Yield |
|---|---|
| With ethanol; barytes Isolieren mit konz.Ueberchlorsaeure; |

-
-
67-48-1
choline chloride

| Conditions | Yield |
|---|---|
| With acid Hydrolysis.Lecithine aus Maiskeimen; | |
| With alkali Hydrolysis.Lecithine aus Maiskeimen; | |
| With acid Hydrolysis.Lecithine aus den Samen von Avena sativa; |

-
-
67-48-1
choline chloride

| Conditions | Yield |
|---|---|
| With alkali Hydrolysis.Lecithine den Samen von Lupinus albus L.; |

-
-
67-48-1
choline chloride

| Conditions | Yield |
|---|---|
| With barium dihydroxide; ethanol |

-
-
67-48-1
choline chloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride Hydrolysis; | |
| With sulfuric acid Hydrolysis; |

| Conditions | Yield |
|---|---|
| at 70 - 80℃; for 0.333333h; | 100% |
| at 120℃; for 0.333333h; Neat (no solvent); | |
| at 74℃; |

| Conditions | Yield |
|---|---|
| at 70 - 80℃; | 100% |
| at 80℃; for 0.5h; | |
| at 50℃; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| In water; acetone at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| at 100℃; | 100% |
| at 20℃; for 24h; | |
| at 50 - 120℃; | |
| at 80℃; |

| Conditions | Yield |
|---|---|
| In acetone for 72h; | 100% |
| In ethanol at 20℃; for 1h; |

| Conditions | Yield |
|---|---|
| at 100℃; | 100% |
| at 50 - 120℃; | |
| at 80 - 100℃; | |
| at 90℃; for 1h; |

| Conditions | Yield |
|---|---|
| In acetonitrile at 85℃; for 24h; | 100% |
-
-
67-48-1
choline chloride

-
-
118072-93-8
1-hydroxy-2-(imidazol-1-yl)ethylidene-1,1-bisphosphonic acid

| Conditions | Yield |
|---|---|
| Stage #1: choline chloride With anion-exchange column A-26 (OH) In methanol Stage #2: 1-hydroxy-2-(imidazol-1-yl)ethylidene-1,1-bisphosphonic acid In methanol; water for 1h; | 100% |
-
-
67-48-1
choline chloride

-
-
118072-93-8
1-hydroxy-2-(imidazol-1-yl)ethylidene-1,1-bisphosphonic acid

| Conditions | Yield |
|---|---|
| Stage #1: choline chloride With anion-exchange column A-26 (OH) In methanol Stage #2: 1-hydroxy-2-(imidazol-1-yl)ethylidene-1,1-bisphosphonic acid In methanol; water for 1h; | 100% |
-
-
67-48-1
choline chloride

-
-
108-29-2
5-methyl-dihydro-furan-2-one

-
-
1211890-41-3
cholinium 4-hydroxyvalerate

| Conditions | Yield |
|---|---|
| In water at 25℃; for 1h; | 99.2% |
-
-
512-56-1
trimethyl phosphite

-
-
67-48-1
choline chloride

-
-
118978-98-6
(2-hydroxyethyl)trimethylammonium dimethyl phosphate

| Conditions | Yield |
|---|---|
| With calcium chloride In toluene at 120℃; for 2h; | 99% |
| In methanol at 80℃; for 12h; |

| Conditions | Yield |
|---|---|
| Stage #1: choline chloride With sodium hydroxide In ethanol at 40℃; for 4h; Stage #2: methanesulfonic acid In ethanol at 0 - 75℃; for 5.5h; | 99% |
-
-
67-48-1
choline chloride

-
-
24801-88-5
3-(triethoxypropyl) isocyanate

| Conditions | Yield |
|---|---|
| With dibutyltin dilaurate In acetonitrile at 80℃; for 14h; Inert atmosphere; | 98.7% |
-
-
75-44-5
phosgene

-
-
67-48-1
choline chloride

-
-
92442-84-7
2-(trimethylammonio)ethyl carbonochloridate chloride

| Conditions | Yield |
|---|---|
| In acetone for 16h; Ambient temperature; | 98% |
| In tetrahydrofuran; toluene | 88% |
| In tetrahydrofuran; toluene for 40h; | |
| With chloroform |

| Conditions | Yield |
|---|---|
| In methanol at 25℃; for 0.5h; | 98% |

-
-
13598-36-2
phosphonic Acid

-
-
16898-52-5
1,3-di(piperidin-4-yl)propane

-
-
67-48-1
choline chloride

-
-
6153-56-6
oxalic acid dihydrate

| Conditions | Yield |
|---|---|
| In water High Pressure; mixt. of diamine, Ga2O3, H3PO3 and choline chloride/oxalic acid (1/1) inH2O was heated in autoclave at 160°C for 1 d; | 97% |

-
-
67-48-1
choline chloride

-
-
15307-79-6
diclofenac sodium

-
-
148439-51-4
(2-hydroxyethyl)tri-methylammonium [o-(2,6-dichloroanilino)phenyl]acetate

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 1h; | 96% |

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 5h; | 95% |
-
-
67-48-1
choline chloride

| Conditions | Yield |
|---|---|
| With chlorosulfonic acid In acetonitrile at 20℃; for 4.25h; | 95% |
| With chlorosulfonic acid In acetonitrile at 5 - 27℃; for 4h; | |
| With chlorosulfonic acid In hexane at 0 - 5℃; for 1.5h; | |
| With chlorosulfonic acid In dichloromethane at 0 - 20℃; for 4h; | |
| With chlorosulfonic acid In acetonitrile at 5℃; |
Choline chloride Chemical Properties
Structure of Choline chloride (CAS NO.67-48-1):
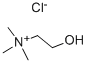
IUPAC Name: 2-hydroxyethyl(trimethyl)azanium chloride
Empirical Formula: C5H14ClNO
Molecular Weight: 139.6238
EINECS: 200-655-4
Melting point: 298-304 ºC
Water solubility: soluble
Sensitive: Hygroscopic
Physical Appearance: White crystalline powder
Product Categories: Quarternary ammonium salts;Ammonium Chlorides (Quaternary);Biochemistry;Quaternary Ammonium Compounds;Vitamin Related Compounds;Vitamins;Antioxidants;Intermediates & Fine Chemicals;Pharmaceuticals
Synonyms of Choline chloride (CAS NO.67-48-1): (2-Hydroxyethyl)trimethylammonium chloride ; (beta-Hydroxyethyl)trimethylammonium chloride ; 2-Hydroxy-N,N,N,-trimethylethanaminium chloride ; Ammonium, (2-hydroxyethyl)trimethyl-, chloride ; Bilineurin chloride ; Biocolina ; Biocoline ; Chloride de choline ; Choline chlorhydrate ; Choline hydrochloride ;
Cholini chloridum ; Cloruro de colina ; Colina cloruro ; Ethanaminium, 2-hydroxy-N,N,N-trimethyl-, chloride ; Hepacholine ;
Hormocline ; Lipotril ; Luridin chloride ; Trimethyl(2-hydroxyethyl)ammonium chloride .
Choline chloride Uses
Choline chloride (CAS NO.67-48-1) is an important additive in feed especially for chicken where it accelerates growth and is mass produced . With urea it forms a deep eutectic solvent. Other commercial choline salts are choline bitartrate choline and hydroxide . In foodstuffs the compound is often present as phosphatidylcholine.
Choline chloride Production
In the industrial Davy process choline chloride is produced from ethylene oxide, hydrochloric acid, and trimethylamine,[1] or from the pre-formed salt:

Choline chloride Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LDLo | intravenous | 25mg/kg (25mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1289, 1935. | |
| dog | LDLo | intravenous | 5mg/kg (5mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1289, 1935. | |
| frog | LDLo | unreported | 1500mg/kg (1500mg/kg) | Proceedings of the Society for Experimental Biology and Medicine. Vol. 51, Pg. 281, 1942. | |
| mouse | LD50 | intraperitoneal | 320mg/kg (320mg/kg) | Proceedings of the Society for Experimental Biology and Medicine. Vol. 51, Pg. 281, 1942. | |
| mouse | LD50 | intravenous | 53mg/kg (53mg/kg) | SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Arzneimittel-Forschung. Drug Research. Vol. 33, Pg. 1016, 1983. |
| mouse | LD50 | oral | 3900mg/kg (3900mg/kg) | SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Arzneimittel-Forschung. Drug Research. Vol. 33, Pg. 1016, 1983. |
| mouse | LDLo | subcutaneous | 735mg/kg (735mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 6, Pg. 477, 1914/1915. | |
| rabbit | LDLo | intraperitoneal | 500mg/kg (500mg/kg) | SENSE ORGANS AND SPECIAL SENSES: CORNEAL DAMAGE: EYE LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Journal of Infectious Diseases. Vol. 42, Pg. 473, 1928. |
| rabbit | LDLo | intravenous | 1100ug/kg (1.1mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: CYANOSIS GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS | Proceedings of the Society for Experimental Biology and Medicine. Vol. 51, Pg. 281, 1942. |
| rabbit | LDLo | rectal | 1gm/kg (1000mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: CYANOSIS GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS | Proceedings of the Society for Experimental Biology and Medicine. Vol. 51, Pg. 281, 1942. |
| rabbit | LDLo | subcutaneous | 1gm/kg (1000mg/kg) | Proceedings of the Society for Experimental Biology and Medicine. Vol. 51, Pg. 281, 1942. | |
| rat | LD50 | intraperitoneal | 450mg/kg (450mg/kg) | Indian Journal of Experimental Biology. Vol. 24, Pg. 91, 1986. | |
| rat | LD50 | oral | 3400mg/kg (3400mg/kg) | SENSE ORGANS AND SPECIAL SENSES: CHROMODACYRORREA: EYE BEHAVIORAL: EXCITEMENT LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Proceedings of the Society for Experimental Biology and Medicine. Vol. 58, Pg. 87, 1945. |
Choline chloride Safety Profile
Hazard Codes:  Xi
Xi
The Risk Statements information of Choline chloride :
36/37/38: Irritating to eyes, respiratory system and skin
The Safety Statements information of Choline chloride :
26: In case of contact with eyes, rinse immediately with plenty of WATER and seek medical advice
36: Wear suitable protective clothing
WGK Germany: 1
RTECS: KH2975000
F: 3-9
Related Products
- Choline
- Choline (1alpha,3alpha,4alpha,5beta)-1,3-bis(3,4-dihydroxycinnamoyloxy)-4,5-dihydroxycyclohexanecarboxylate
- Choline acetate (ester)
- Choline bicarbonate
- Choline bitartrate
- Choline chloride
- Choline dihydrogencitrate salt
- Choline fenofibrate
- Choline glycerophosphate
- Choline hydroxide
- 67482-48-8
- 674-82-8
- 67482-93-3
- 67483-13-0
- 67485-29-4
- 67488-50-0
- 6748-91-0
- 67491-43-4
- 67492-50-6
- 674-97-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View