-
Name
Tadalafil
- EINECS 200-835-2
- CAS No. 171596-29-5
- Article Data48
- CAS DataBase
- Density 1.51 g/cm3
- Solubility
- Melting Point 298-300 °C
- Formula C22H19N3O4
- Boiling Point 679.1 °C at 760 mmHg
- Molecular Weight 389.411
- Flash Point 364.5 °C
- Transport Information
- Appearance white to off-white cyrstalline solid
- Safety 16-36/37
- Risk Codes 11-20/21/22-36
-
Molecular Structure
- Hazard Symbols F,Xn
- Synonyms Pyrazino[1',2':1,6]pyrido[3,4-b]indole-1,4-dione,6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-, (6R-trans)-;(6R,12aR)-6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methylpyrazino[1',2':1,6]pyrido[3,4-b]indole-1,4-dione;GF 196960;Tadalafil [USAN];Tildenafil;Calais;(6R-trans)-6-(1,3-Benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-pyrazino(1',2':1,6)pyrido(3,4-b)indole-1,4-dione;Adcirca;
- PSA 74.87000
- LogP 2.08710
Synthetic route

-
-
74-89-5
methylamine

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 5℃; for 3h; Temperature; Solvent; | 99.3% |
-
-
74-89-5
methylamine

-
-
171489-59-1
methyl (1R,3R)-1-(3,4-methylenedioxyphenyl)-2-chloroacetyl-2,3,4,9-tetrahydro-9H-pyrido[3,4-b]indol-3-carboxylate

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| In water; N,N-dimethyl-formamide at 20℃; | 95% |
| In N,N-dimethyl-formamide at 20℃; for 10h; | 95% |
| In methanol for 3h; Reflux; Large scale; | 95.3% |

-
-
74-95-3
1,2-dibromomethane

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| With sodium hydroxide In N,N-dimethyl-formamide at 60℃; for 6h; | 95% |
| With caesium carbonate In N,N-dimethyl-formamide at 80℃; for 8h; | 93% |
| With caesium carbonate In N,N-dimethyl-formamide at 80℃; for 8h; | 93% |
| With caesium carbonate In N,N-dimethyl-formamide at 80℃; for 8h; | 93% |
-
-
1224724-00-8
(1R,3R)-methyl-1,2,3,4-tetrahydro-2-(2-(benzyl(methyl)amino)acetyl)-1-(3,4-methylenedioxyphenyl)-9H-pyrido[3,4-b]indole-3-carboxylate

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| With hydrogen; Raney Ni In ISOPROPYLAMIDE at 80℃; under 2280.15 Torr; for 22h; Product distribution / selectivity; | 94% |
-
-
171489-59-1
methyl (1R,3R)-1-(3,4-methylenedioxyphenyl)-2-chloroacetyl-2,3,4,9-tetrahydro-9H-pyrido[3,4-b]indol-3-carboxylate

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| With methylamine In dichloromethane for 2h; | 93.5% |
| With methylamine In water for 20h; Ionic liquid; | 355 mg |
-
-
52605-49-9
sarcosine ethyl ester hydrochloride


-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethylene glycol at 110℃; Solvent; Reagent/catalyst; Temperature; Green chemistry; | 92.7% |
-
-
749864-18-4
1-benzo[1,3]dioxol-5-yl-2-{[(9H-fluoren-9-ylmethoxycarbonyl)-methyl-amino]-acetyl}-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylic acid methyl ester

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| With piperidine In N,N-dimethyl-formamide at 20℃; for 1h; | 92% |

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| Stage #1: C22H20N4O3 With sulfuric acid In isopropyl alcohol for 12h; Reflux; Stage #2: With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 8h; Temperature; | 91.2% |

-
-
74-89-5
methylamine

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; water at 50℃; Solvent; Temperature; | 91% |
-
-
107-97-1
sarcosine


-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| Stage #1: sarcosine; (1R,3R)-1-benzo[1,3]dioxol-5-yl-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylic acid methyl ester hydrochloride With triethylamine In tetrahydrofuran at 30℃; for 0.333333h; Stage #2: With dicyclohexyl-carbodiimide In tetrahydrofuran for 0.5h; Solvent; Reagent/catalyst; Temperature; Further stages; | 90.54% |
-
-
120-57-0
piperonal

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 80℃; for 16h; Solvent; Temperature; | 58.2% |
-
-
74-89-5
methylamine

-
-
171489-59-1
methyl (1R,3R)-1-(3,4-methylenedioxyphenyl)-2-chloroacetyl-2,3,4,9-tetrahydro-9H-pyrido[3,4-b]indol-3-carboxylate

-
A
-
171596-27-3
(6R,12aS)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

-
B
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| In methanol at 50℃; for 16h; | A 1.1% B 54% |

-
-
474668-76-3
(1R,3R)-1,2,3,4-tetrahydro-1-(3,4-methylenedioxyphenyl)-9H-pyrido[3,4-b]indole-3-carboxylic acid hydrochloride

-
-
52605-49-9
sarcosine ethyl ester hydrochloride

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; triethylamine; dicyclohexyl-carbodiimide In N,N-dimethyl-formamide at 50 - 55℃; for 10h; Large scale reaction; | 52.6% |
-
-
951661-81-7
(1R,3R)-1-(benzo[d][1,3]dioxol-5-yl)-2-(2-chloroacetyl)-N-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxamide

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| Stage #1: (1R,3R)-1-(benzo[d][1,3]dioxol-5-yl)-2-(2-chloroacetyl)-N-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxamide With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -50 - -35℃; for 2 - 6h; Stage #2: With ammonium chloride In tetrahydrofuran; hexane; water; ethyl acetate at -40 - 30℃; Product distribution / selectivity; | 48% |
| Stage #1: (1R,3R)-1-(benzo[d][1,3]dioxol-5-yl)-2-(2-chloroacetyl)-N-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxamide With n-butyllithium In tetrahydrofuran; hexane at -40 - -35℃; for 2.5h; Stage #2: With ammonium chloride In tetrahydrofuran; hexane; water; ethyl acetate at -40 - 30℃; Product distribution / selectivity; |
-
-
120-57-0
piperonal

-
-
5241-64-5
Boc-D-Trp-OH

-
-
79-04-9
chloroacetyl chloride

-
-
74-89-5
methylamine

-
A
-
171596-28-4
(6S,12aR)-6-(benzo[d][1,3]dioxol-5-yl)-2-methyl-2,3,12,12a tetrahydropyrazino [1',2':1,6] pyrido[3,4b] indole1,4(6H,7H)-dione

-
B
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| Stage #1: Boc-D-Trp-OH With dmap; dicyclohexyl-carbodiimide In dichloromethane for 0.166667h; Microwave irradiation; Stage #2: piperonal With trifluoroacetic acid In chloroform for 0.5h; Pictet-Spengler cyclisation; Microwave irradiation; Stage #3: chloroacetyl chloride; methylamine Further stages; | A 45% B 32% |

-
-
120-57-0
piperonal

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 25 percent / TFA / methanol; CH2Cl2 / 60 h / 20 °C 2: 91 percent / sodium bicarbonate / CH2Cl2 / 1 h / 0 °C 3: 54 percent / methanol / 16 h / 50 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 85 percent / aq.HCl / methanol / 36 h / Heating 2: 78 percent / NaHCO3 / CH2Cl2; H2O 3: 88 percent / CHCl3; ethanol / 7 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: 95 percent / Et3N; MgSO4 / CH2Cl2 / 24 h 2: 43 percent / DMAP / CH2Cl2 / 2 h / 20 °C 3: 92 percent / methanol / 16 h / 50 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 95 percent / Et3N; MgSO4 / CH2Cl2 / 24 h 2: DMAP; basic alumina / CH2Cl2 / 2 h / -25 - 20 °C 3: 92 percent / piperidine / dimethylformamide / 1 h / 20 °C View Scheme |
-
-
14907-27-8, 5619-09-0, 7524-52-9, 26988-71-6, 41222-70-2, 67557-19-1, 109492-62-8
(R)-(+)-tryptophan methyl ester hydrochloride

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 25 percent / TFA / methanol; CH2Cl2 / 60 h / 20 °C 2: 91 percent / sodium bicarbonate / CH2Cl2 / 1 h / 0 °C 3: 54 percent / methanol / 16 h / 50 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 85 percent / aq.HCl / methanol / 36 h / Heating 2: 78 percent / NaHCO3 / CH2Cl2; H2O 3: 88 percent / CHCl3; ethanol / 7 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: 95 percent / Et3N; MgSO4 / CH2Cl2 / 24 h 2: 43 percent / DMAP / CH2Cl2 / 2 h / 20 °C 3: 92 percent / methanol / 16 h / 50 °C View Scheme |
-
-
171596-41-1
(1R,3R)-methyl 1,2,3,4-tetrahydro-1-(3,4-methylenedioxyphenyl)-9H-pyrido[3,4-b]indole-3-carboxylate

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 91 percent / sodium bicarbonate / CH2Cl2 / 1 h / 0 °C 2: 54 percent / methanol / 16 h / 50 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 93 percent / NaHCO3 / CHCl3 / 20 °C 2: 77 percent / ethanol / Heating View Scheme |

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 78 percent / NaHCO3 / CH2Cl2; H2O 2: 88 percent / CHCl3; ethanol / 7 h / Heating View Scheme |

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 43 percent / DMAP / CH2Cl2 / 2 h / 20 °C 2: 92 percent / methanol / 16 h / 50 °C View Scheme | |
| Multi-step reaction with 2 steps 1: DMAP; basic alumina / CH2Cl2 / 2 h / -25 - 20 °C 2: 92 percent / piperidine / dimethylformamide / 1 h / 20 °C View Scheme |
-
-
120-57-0
piperonal

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 42 percent / TFA / CH2Cl2 / 20 °C 2: 93 percent / NaHCO3 / CHCl3 / 20 °C 3: 77 percent / ethanol / Heating View Scheme |
-
-
4299-70-1, 7303-49-3, 22032-65-1
D-Tryptophan methyl ester

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 42 percent / TFA / CH2Cl2 / 20 °C 2: 93 percent / NaHCO3 / CHCl3 / 20 °C 3: 77 percent / ethanol / Heating View Scheme | |
| Multi-step reaction with 3 steps 1.1: hydrogenchloride / water; toluene / 15 h / Reflux; Large scale 2.1: triethylamine / dichloromethane / 0.17 h / 20 °C / Large scale 2.2: 1 h / 20 °C / Large scale 3.1: methanol / 3 h / Reflux; Large scale View Scheme | |
| Multi-step reaction with 3 steps 1: isopropyl alcohol / 10 h / 70 - 80 °C / Large scale 2: triethylamine / chloroform / 2 h / 20 - 30 °C 3: methylamine / dichloromethane / 2 h View Scheme |
-
-
1321600-91-2
methyl 1-(benzo[d][1,3]dioxol-5-yl)-2-(2-chloroacetyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate

-
-
593-51-1
methylamine hydrochloride

-
A
-
171596-28-4
(6S,12aR)-6-(benzo[d][1,3]dioxol-5-yl)-2-methyl-2,3,12,12a tetrahydropyrazino [1',2':1,6] pyrido[3,4b] indole1,4(6H,7H)-dione

-
B
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| With triethylamine In methanol Reflux; |

-
-
120-57-0
piperonal

-
-
14907-27-8, 5619-09-0, 7524-52-9, 26988-71-6, 41222-70-2, 67557-19-1, 109492-62-8
(R)-(+)-tryptophan methyl ester hydrochloride

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 3 h / 85 °C 1.2: 1 h / 0 - 10 °C 2.1: water; tetrahydrofuran / 1 h / 55 °C / Inert atmosphere View Scheme |
-
-
120-57-0
piperonal

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 0.25 h / 110 °C 2: triethylamine / water; tetrahydrofuran / 2 h / 0 - 10 °C / Inert atmosphere 3: water; tetrahydrofuran / 1 h / 55 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: 0.25 h / 110 °C 2: triethylamine / tetrahydrofuran; water / 2 h / 0 - 10 °C / Inert atmosphere 3: tetrahydrofuran; water / 1 h / 55 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: acetonitrile / 105 °C / Autoclave 2: sodium carbonate / acetonitrile; water / 5 - 10 °C 3: water; isopropyl alcohol / 110 - 120 °C View Scheme |

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 4-methyl-morpholine / tetrahydrofuran / Inert atmosphere 1.2: 1 h / -20 - 20 °C / Inert atmosphere 2.1: trifluoroacetic acid / toluene / 15 h / 45 - 50 °C 3.1: toluene / 8 h / 110 °C View Scheme |

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| In toluene at 110℃; for 8h; | 0.39 g |
-
-
120-57-0
piperonal

-
-
1422164-52-0
(R)-ethyl 2-(2-((tert-butoxycarbonyl)amino)-3-(1H-indol-3-yl)-N-methylpropanamido)acetate

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: trifluoroacetic acid / toluene / 15 h / 45 - 50 °C 2: toluene / 8 h / 110 °C View Scheme |
-
-
5241-64-5
Boc-D-Trp-OH

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 4-methyl-morpholine / tetrahydrofuran / 0.33 h / -20 °C / Inert atmosphere 1.2: 0.5 h / -20 °C / Inert atmosphere 2.1: 4-methyl-morpholine / tetrahydrofuran / Inert atmosphere 2.2: 1 h / -20 - 20 °C / Inert atmosphere 3.1: trifluoroacetic acid / toluene / 15 h / 45 - 50 °C 4.1: toluene / 8 h / 110 °C View Scheme |
-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

-
-
171596-27-3
(6R,12aS)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dimethyl sulfoxide; isopropyl alcohol at 83℃; for 5h; | 98% |
-
-
81-04-9
naphthalene-1,5-disulfonate

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| In Isopropyl acetate at 45℃; for 48h; pH=2 - 3; Solvent; | 95.4% |
-
-
69-72-7
salicylic acid

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| In Isopropyl acetate at 45℃; for 48h; pH=2 - 3; Solvent; | 94.81% |
-
-
17199-29-0
(S)-Mandelic acid

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| In Isopropyl acetate at 45℃; for 48h; pH=2 - 3; Solvent; | 94.25% |
-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

-
-
1021702-43-1
(6R,12aS)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-3,3,12a-trideuterio-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| With water-d2; potassium carbonate In tetrahydrofuran; dimethylsulfoxide-d6 at 65℃; for 16h; | 94% |
-
-
141-82-2
malonic acid

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

-
-
1242099-10-0
C3H4O4*C22H19N3O4

| Conditions | Yield |
|---|---|
| In ethyl acetate for 5h; | 85% |
| In acetonitrile Product distribution / selectivity; | |
| In acetonitrile at 25℃; for 0.5h; |
-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

-
-
1220393-12-3
3-((2-(benzo[d][1,3]dioxole-5-carbonyl)-1H-indol-3-yl)methyl)-1-methylpiperazine-2,5-dione

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; water; tetramethlyammonium chloride In dimethyl sulfoxide at 40℃; for 7h; | 79% |
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In tetrahydrofuran; water at 20℃; for 8h; Product distribution / selectivity; |
-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; acetic acid at 50℃; for 5h; Reagent/catalyst; | 78% |
-
-
696-63-9
4-Methoxybenzenethiol

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| With tetrabutylammonium tetrafluoroborate for 4.5h; Electrolysis; Inert atmosphere; Sealed tube; regioselective reaction; | 65% |
-
-
456-24-6
2-Fluoro-5-nitropyridine

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 23℃; for 40h; Inert atmosphere; | 61% |
-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| With (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; deuterium In tetrahydrofuran at 55℃; under 750.075 Torr; for 22h; Inert atmosphere; | 46% |

-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| With [(Tp)NiIV(CF3)3] In dimethyl sulfoxide at 25℃; for 24h; Inert atmosphere; Sealed tube; Glovebox; | 43% |
-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite; 2,3-dicyano-5,6-dichloro-p-benzoquinone In acetonitrile at 20℃; for 15h; Irradiation; | 42% |
-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

| Conditions | Yield |
|---|---|
| With potassium superoxide; 18-crown-6 ether In N,N-dimethyl-formamide at 25℃; for 6h; Winterfeldt oxidation; | 38% |
| Multi-step reaction with 2 steps 1: O2; KOtBu / dimethylformamide; tetrahydrofuran / 4 h 2: 16.2 mg / PyBrOP; di-isopropylethylamine / dimethylformamide; tetrahydrofuran / 16 h / 25 °C View Scheme |
-
-
171596-29-5
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione

-
-
422311-80-6
(6R,12aR)-6-benzo[1,3]dioxol-5-yl-2-methyl-2,3,6,7,12,12a-hexahydropyrazino[1',2':1,6] pyrido[3,4-b]indole-1,4-dithione

| Conditions | Yield |
|---|---|
| With Lawessons reagent In tetrahydrofuran at 20℃; for 72h; | 25% |
Cialis Chemical Properties
The Molecular Structure of Tadalafil (CAS NO.171596-29-5):
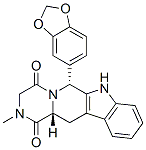
Empirical Formula: C22H19N3O4
Molecular Weight: 389.404
Appearance: Off-White Cyrstalline Solid
Nominal Mass: 389 Da
Average Mass: 389.404 Da
Monoisotopic Mass: 389.137556 Da
Index of Refraction: 1.758
Molar Refractivity: 105.27 cm3
Molar Volume: 256.2 cm3
Surface Tension: 81.5 dyne/cm
Density: 1.51 g/cm3
Flash Point: 364.5 °C
Enthalpy of Vaporization: 99.67 kJ/mol
Boiling Point: 679.1 °C at 760 mmHg
Vapour Pressure: 2.67E-18 mmHg at 25°C
Melting Point: 298-300°C
Product Categories: Active Pharmaceutical Ingredients;Cnbio;Erectile Dysfunction;Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals;API's
InChI: InChI=1/C22H19N3O4/c1-24-10-19(26)25-16(22(24)27)9-14-13-4-2-3-5-15(13)23-20(14)21(25)12-6-7-17-18(8-12)29-11-28-17/h2-8,16,21,23H,9-11H2,1H3/t16-,21-/m1/s1
Smiles: C1N(C([C@H]2Cc3c([C@H](N2C1=O)c1ccc2c(c1)OCO2)[nH]c1ccccc31)=O)C
Cialis History
Tadalafil (CAS NO.171596-29-5) was discovered by Glaxo Wellcome (now GlaxoSmithKline) under a partnership between Glaxo and ICOS to develop new drugs that began in August 1991. In December 2003, after sildenafil (Viagra) and vardenafil (Levitra),the Food and Drug Administration approved tadalafil (as Cialis) for sale in the United States as the third ED prescription drug pill. Then in May 2009,Tadalafil was approved in the United States for the treatment of pulmonary arterial hypertension and is currently under regulatory review in other regions for this condition.
Cialis Uses
Tadalafil (CAS NO.171596-29-5) can be used for the treatment of erectile dysfunction under the name Cialis. Recently Tadalafil (CAS NO.171596-29-5) has been approved for the treatment of pulmonary arterial hypertension and other onditions.
Cialis Safety Profile
The most common side effects of tadalafil are: are headache, indigestion, flushing,back pain, muscle aches and stuffy or runny nose. These side effects reflect the ability of PDE5 inhibition to vasodilate (cause blood vessels to widen), these usually go away after a few hours. Back pain and muscle aches maybe occur 12 to 24 hours after taking the drug,and the symptom usually disappears after 48 hours.
Cialis Specification
Tadalafil (CAS NO.171596-29-5) is the main raw materials of Cialis,compare with Sildenafil,Tadalafil have less side effects, after taking two days,30% of people have muscles ache feeling (mainly waist and legs). It is also called as Tadalafil [USAN] ; (6R,12aR)-2,3,6,7,12,12a-Hexahydro-2-methyl-6-(3,4-(methylenedioxy)phenyl) pyrazino(1',2':1,6)pyrido(3,4-b)indole-1,4-dione ; (6R,12aR)-2,3,6,7,12,12a-Hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)pyrazino(1',2':1,6)pyrido(3,4-b)indole-1,4-dione ; (6R-trans)-6-(1,3-Benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-pyrazino(1',2':1,6)pyrido(3,4-b)indole-1,4-dione ; Adcirca ; Cialis ; Tadalafil ; Tadalafil Lilly ;Pyrazino(1',2':1,6)pyrido(3,4-b)indole-1,4-dione, 6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-, (6R,12aR)- ; Pyrazino(1',2':1,6)pyrido(3,4-b)indole-1,4-dione, 6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-, (6R-trans)- .
Related Products
- Cialis
- 17159-79-4
- 17159-80-7
- 171599-83-0
- 17160-89-3
- 1716-12-7
- 17161-33-0
- 17162-29-7
- 17162-39-9
- 1716-42-3
- 1716-43-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View