-
Name
Dimethyl (2-oxo-4-phenylbutyl)phosphonate
- EINECS
- CAS No. 41162-19-0
- Article Data40
- CAS DataBase
- Density 1.152 g/cm3
- Solubility
- Melting Point 120-122 (0.5 MMHG)oC
- Formula C12H17O4P
- Boiling Point 362.7 °C at 760 mmHg
- Molecular Weight 256.238
- Flash Point 186.8 °C
- Transport Information
- Appearance clear yellow liquid
- Safety 24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Phosphonicacid, (2-oxo-4-phenylbutyl)-, dimethyl ester (9CI);(2-Oxo-4-phenylbutyl)phosphonic acid dimethyl ester;Dimethyl(2-oxo-4-phenylbutyl)phosphonate;
- PSA 62.41000
- LogP 2.67420
Synthetic route
-
-
103-25-3
3-phenylpropanoic acid methyl ester

-
-
756-79-6
dimethyl methane phosphonate

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl methane phosphonate With n-butyllithium In tetrahydrofuran at -78℃; for 0.5h; Inert atmosphere; Stage #2: 3-phenylpropanoic acid methyl ester In tetrahydrofuran at -78℃; for 1h; | 98% |
| With n-butyllithium In tetrahydrofuran at -78 - 0℃; Inert atmosphere; | 98% |
| Stage #1: dimethyl methane phosphonate With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.5h; Stage #2: 3-phenylpropanoic acid methyl ester In tetrahydrofuran; hexane at -78 - 0℃; | 97% |
-
-
2021-28-5
ethyl dihydrocinnamate

-
-
756-79-6
dimethyl methane phosphonate

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| With n-butyllithium; diisopropylamine In tetrahydrofuran at 5 - 20℃; Inert atmosphere; | 95% |
| With n-butyllithium 1.) THF, -78 deg C, 15 min, 2.) THF, -78 deg C, 30 min; Multistep reaction; | |
| With n-butyllithium In tetrahydrofuran; diethyl ether; hexane; water; acetic acid | |
| Stage #1: dimethyl methane phosphonate With n-hexyllithium In tetrahydrofuran; hexane at -80℃; Inert atmosphere; Stage #2: ethyl dihydrocinnamate In tetrahydrofuran; hexane at 20℃; Inert atmosphere; Stage #3: With water In tetrahydrofuran; hexane at 20℃; | |
| With n-butyllithium In tetrahydrofuran; hexane |

| Conditions | Yield |
|---|---|
| With phosphorous acid trimethyl ester In acetonitrile | 85% |
-
-
352276-27-8
1-iodo-4-phenylbutan-2-one

-
-
121-45-9
phosphorous acid trimethyl ester

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 2.5h; Reflux; Inert atmosphere; | 60% |
-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
100-44-7
benzyl chloride

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| (i) NaH, THF, (ii) nBuLi, hexane, (iii) /BRN= 471308/; Multistep reaction; |
-
-
140-10-3
(E)-3-phenylacrylic acid

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: H2 / 5 percent Pd/C / ethyl acetate 2.1: MeOH 3.1: N-BuLi / tetrahydrofuran / 0 °C 3.2: tetrahydrofuran View Scheme |
-
-
501-52-0
3-Phenylpropionic acid

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: MeOH 2.1: N-BuLi / tetrahydrofuran / 0 °C 2.2: tetrahydrofuran View Scheme | |
| Multi-step reaction with 2 steps 1: sulfuric acid 2: n-butyllithium / tetrahydrofuran View Scheme | |
| Multi-step reaction with 2 steps 1.1: sulfuric acid / 2 h / Reflux 2.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 2.2: 1 h / -78 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sulfuric acid / 2 h / Reflux 2: n-butyllithium / tetrahydrofuran / -78 - 0 °C / Inert atmosphere View Scheme |
-
-
2550-26-7
4-Phenyl-2-butanone

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: bromine / methanol / 2.83 h / -5 - 20 °C / Inert atmosphere 2: sodium iodide / acetone / 6 h / 20 °C / Inert atmosphere 3: acetonitrile / 2.5 h / 20 °C / Reflux; Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: bromine / methanol / 4 h / 0 - 15 °C 2: sodium iodide / acetone / 12 h 3: acetonitrile / 3 h / 65 °C View Scheme | |
| Multi-step reaction with 2 steps 1: bromine; acetic acid / methanol / Inert atmosphere 2: acetonitrile / 3 h / 65 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: iodine; copper(II) oxide / methanol; dichloromethane / 15 h / 42 °C / Inert atmosphere 2: acetonitrile / 3 h / 65 °C / Inert atmosphere View Scheme |
-
-
31984-10-8
1-bromo-4-phenylbutan-2-one

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium iodide / acetone / 6 h / 20 °C / Inert atmosphere 2: acetonitrile / 2.5 h / 20 °C / Reflux; Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: sodium iodide / acetone / 12 h 2: acetonitrile / 3 h / 65 °C View Scheme |
-
-
512-56-1
trimethyl phosphite

-
-
352276-27-8
1-iodo-4-phenylbutan-2-one

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| In acetonitrile at 65℃; for 3h; | 73 g |
| In acetonitrile at 65℃; for 3h; Inert atmosphere; |
-
-
512-56-1
trimethyl phosphite

-
-
31984-10-8
1-bromo-4-phenylbutan-2-one

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| In acetonitrile at 65℃; for 3h; Inert atmosphere; | 72 g |
-
-
768-56-9
4-Phenyl-1-butene

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 20 °C / Cooling with ice 2: zinc(II) chloride / toluene / 4 h / 65 - 70 °C / Inert atmosphere 3: Jones reagent / acetone / 5 h / Cooling with ice View Scheme |

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| With Jones reagent In acetone for 5h; Cooling with ice; | 15.8 g |
-
-
64091-14-1
(3aR,4R,5R,6aS)-5-((tert-butyldimethylsilyl)oxy)-2-oxohexahydro-2H-cyclopenta[b]furan-4-carbaldehyde

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

-
-
1240483-13-9
(3aR,4R,5R,6aS)-5-((tert-butyldimethylsilyl)oxy)-4-((E)-3-oxo-5-phenylpent-1-en-1-yl)hexahydro-2H-cyclopenta[b]furan-2-one

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonate With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; Inert atmosphere; Stage #2: (3aR,4R,5R,6aS)-5-((tert-butyldimethylsilyl)oxy)-2-oxohexahydro-2H-cyclopenta[b]furan-4-carbaldehyde In tetrahydrofuran; mineral oil at 0℃; | 93% |
| With triethylamine; lithium chloride In acetonitrile for 2h; Inert atmosphere; | 92% |
-
-
64091-14-1
(3aR,4R,5R,6aS)-5-((tert-butyldimethylsilyl)oxy)-2-oxohexahydro-2H-cyclopenta[b]furan-4-carbaldehyde

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonate With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 1h; Inert atmosphere; Stage #2: (3aR,4R,5R,6aS)-5-((tert-butyldimethylsilyl)oxy)-2-oxohexahydro-2H-cyclopenta[b]furan-4-carbaldehyde In tetrahydrofuran; mineral oil at 0℃; for 1.5h; | 93% |
| Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonate With potassium carbonate In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Stage #2: (3aR,4R,5R,6aS)-5-((tert-butyldimethylsilyl)oxy)-2-oxohexahydro-2H-cyclopenta[b]furan-4-carbaldehyde In tetrahydrofuran at -20℃; Reagent/catalyst; Inert atmosphere; | 86% |
-
-
104-53-0
3-phenyl-propionaldehyde

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

-
-
79559-59-4
(4E)-1,7-diphenylhept-4-en-3-one

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonate With potassium carbonate In tetrahydrofuran at 20℃; for 0.666667h; Inert atmosphere; Stage #2: 3-phenyl-propionaldehyde at 40℃; for 12h; | 92% |
| With potassium hexamethylsilazane Multistep reaction; |
-
-
104-53-0
3-phenyl-propionaldehyde

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

-
-
79559-59-4
1,7-diphenyl-4-heptene-3-one

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonate With potassium carbonate In tetrahydrofuran at 20℃; for 0.666667h; Inert atmosphere; Stage #2: 3-phenyl-propionaldehyde In tetrahydrofuran at 40℃; for 12h; | 92% |
-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

-
-
38754-71-1
(3aR,4R,5R,6aS)-4-formyl-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl biphenyl-4-carboxylate

-
-
41639-72-9
(1S,5R,6R,7R)-6-(3-oxo-5-phenyl-1E-pentenyl)-7-<(4-phenylbenzoyl)oxy>-2-oxabicyclo<3.3.0>octan-3-one

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonate With potassium carbonate In isopropyl alcohol at 25℃; for 0.5h; Horner-Wadsworth-Emmons Olefination; Stage #2: (3aR,4R,5R,6aS)-4-formyl-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl biphenyl-4-carboxylate In isopropyl alcohol at 25℃; for 5h; Temperature; Horner-Wadsworth-Emmons Olefination; | 91% |
| With N-ethyl-N,N-diisopropylamine; lithium chloride In acetonitrile at -15℃; | 70% |
| With sodium hydride 1.) THF, 90 min, 2.) THF, 30 min; Multistep reaction; | |
| With sodium hydroxide In dichloromethane; water at 0 - 5℃; for 1.5h; Product distribution / selectivity; |
-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

-
-
62961-72-2
Corey aldehyde

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonate With potassium tert-butylate In tetrahydrofuran at 0℃; for 0.5h; Inert atmosphere; Stage #2: Corey aldehyde In tetrahydrofuran at 20℃; for 6h; Reagent/catalyst; Temperature; Inert atmosphere; | 88% |
| Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonate With sodium hydride In tetrahydrofuran at 0℃; for 1h; Inert atmosphere; Stage #2: Corey aldehyde In tetrahydrofuran at 0 - 20℃; for 6h; Inert atmosphere; |

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| With sodium hexamethyldisilazane at 0℃; for 12h; Horner-Wadsworth-Emmons olefination; | 87% |

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonate With sodium hydride In tetrahydrofuran at 20℃; for 1h; Horner-Wadsworth-Emmons olefination; Stage #2: C8H13NO3 In tetrahydrofuran at 20℃; for 1h; Horner-Wadsworth-Emmons olefination; | 86% |
-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

-
-
39746-01-5
(1S,5R,6R,7R)-6-formyl-7-benzoyloxy-2-oxabicyclo[3.3.0]octan-3-one

-
-
55076-60-3
(3aR,4R,5R,6aS)-2-oxo-4-((1E)-3-oxo-5-phenylpent-1-en-1-yl)-hexahydro-2H-cyclopenta[b]furan-5-yl benzoate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonate With N-ethyl-N,N-diisopropylamine; lithium chloride In acetonitrile at -15℃; Stage #2: (1S,5R,6R,7R)-6-formyl-7-benzoyloxy-2-oxabicyclo[3.3.0]octan-3-one In acetonitrile at -15℃; Wittig Reaction; | 84.7% |
| With triethylamine; lithium chloride In tetrahydrofuran; dichloromethane at 0℃; for 2h; Horner-Wadsworth-Emmons Olefination; Cooling with ice; | 73% |
| Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonate With sodium hexamethyldisilazane In 1,2-dimethoxyethane Metallation; Stage #2: (1S,5R,6R,7R)-6-formyl-7-benzoyloxy-2-oxabicyclo[3.3.0]octan-3-one In 1,2-dimethoxyethane Condensation; | |
| With n-butyllithium In tetrahydrofuran; hexane; dichloromethane; acetic acid | |
| With triethylamine; lithium chloride In dichloromethane at 0℃; for 2h; Horner-Emmons reaction; |
-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

-
-
563-96-2
2,2-dihydroxyacetic acid

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at -20℃; for 2h; Horner-Wadsworth-Emmons Olefination; stereoselective reaction; | 83% |
-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

-
-
850209-99-3
2-dibenzylamino-5-oxo-pentanoic acid benzyl ester

-
-
1379764-51-8
(S,E)-benzyl 2-(dibenzylamino)-7-oxo-9-phenylnon-5-enoate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonate With N-ethyl-N,N-diisopropylamine; lithium chloride In acetonitrile at 20℃; Horner-Wadsworth-Emmons olefination; Inert atmosphere; Stage #2: 2-dibenzylamino-5-oxo-pentanoic acid benzyl ester In acetonitrile at 20℃; Horner-Wadsworth-Emmons olefination; Inert atmosphere; | 80% |
-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; lithium chloride In acetonitrile at 20℃; for 6h; Horner-Wadsworth-Emmons Olefination; Inert atmosphere; | 73% |
-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate


-
-
1395619-06-3
(3aR,4R,5R,6aS)-5-(tert-butyldiphenylsilyloxy)-4-((E)-3-oxo-5-phenylpent-1-enyl)hexahydro-2H-cyclopenta[b]furan-2-one

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonate With triethylamine; lithium chloride In tetrahydrofuran at -10℃; for 1.5h; Horner-Wadsworth-Emmons Olefination; Inert atmosphere; Stage #2: (3aR,4R,5R,6aS)-5-((tert-butyldiphenylsilyl)oxy)-2-oxohexahydro-2H-cyclopenta[b]furan-4-carbaldehyde In tetrahydrofuran at -10℃; for 2.41667h; Inert atmosphere; | 72% |

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonate With sodium hydride In 1,2-dimethoxyethane at -5 - 25℃; Inert atmosphere; Stage #2: C30H34O7 In 1,2-dimethoxyethane at -5 - 25℃; Wittig reaction; Inert atmosphere; | 70% |

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

-
-
7646-69-7
sodium hydride

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonate; sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 0.166667h; Inert atmosphere; Stage #2: [(S)-2-(6-oxo-3,6-dihydro-2H-pyran-2-yl) acetaldehyde] In tetrahydrofuran; mineral oil at -78℃; for 1h; Inert atmosphere; | 66% |

-
-
163074-98-4
[(3aR,4R,5R,6aS)-hexahydro-2-oxo-5-(phenylmethoxy)-2H-cyclopenta[b]furan-4-carboxaldehyde]

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| With triethylamine; lithium chloride In tetrahydrofuran at -18 - -10℃; for 25h; Horner-Wadsworth-Emmons Olefination; | 65% |
-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

-
-
88284-48-4
2-(trimethylsilyl)phenyl trifluoromethanesulfonate

-
-
1170736-73-8
dimethyl 2-(3-phenylpropanoyl)benzylphosphonate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonate; 2-(trimethylsilyl)phenyl trifluoromethanesulfonate With cesium fluoride In tetrahydrofuran at 60℃; for 67h; Inert atmosphere; Stage #2: With silica gel In tetrahydrofuran; ethyl acetate | 65% |

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonate With sodium hydride In 1,2-dimethoxyethane at -5 - 25℃; for 1.25h; Wittig reaction; Inert atmosphere; Stage #2: (Z)-4-formyl-5-(7-isopropoxy-7-oxohept-2-enyl)cyclopentane-1,3-diyl dibenzoate In 1,2-dimethoxyethane at -5 - 25℃; for 2h; Wittig reaction; Inert atmosphere; | 65% |
-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| With 4-toluenesulfonyl azide; potassium carbonate | 63% |
-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

-
-
128948-10-7
(-)-7α-triethylsilyloxy-6β-formyl-cis-2-oxabicyclo<3.3.0>octan-3-one

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonate With triethylamine; lithium chloride In tetrahydrofuran at -15 - -5℃; Stage #2: (-)-7α-triethylsilyloxy-6β-formyl-cis-2-oxabicyclo<3.3.0>octan-3-one In tetrahydrofuran for 1h; | 51% |
| Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonate With lithium hydroxide monohydrate In tert-butyl methyl ether at 20℃; for 1h; Stage #2: (-)-7α-triethylsilyloxy-6β-formyl-cis-2-oxabicyclo<3.3.0>octan-3-one In tert-butyl methyl ether; water at 5℃; for 0.916667h; Wittig-Horner Reaction; | 27.5 g |
| With lithium hydroxide monohydrate In tert-butyl methyl ether at 5℃; for 0.75h; Wittig-Horner Reaction; Inert atmosphere; |
-
-
383-63-1
ethyl trifluoroacetate,

-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

-
-
100-58-3
phenylmagnesium bromide

| Conditions | Yield |
|---|---|
| Stage #1: ethyl trifluoroacetate,; phenylmagnesium bromide In diethyl ether; water at -80 - 0℃; for 2h; Inert atmosphere; Stage #2: dimethyl(2-oxo-4-phenylbutyl)phosphonate With triethylamine; lithium bromide In tetrahydrofuran; diethyl ether; water at 0 - 40℃; for 5.16h; Inert atmosphere; | A 51% B 4% |


-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran Horner-Wadsworth-Emmons reaction; | 20% |
-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

-
-
104596-33-0
methyl <1α,2α(Z),3α,4α>-7-<3-formyl-7-oxabicyclo<2.2.1>hept-2-yl>-5-heptenoate

| Conditions | Yield |
|---|---|
| With sodium hydride 1.) DME, 2.) 23 deg C; Multistep reaction; |
-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate


| Conditions | Yield |
|---|---|
| With triethylamine; lithium bromide 1.) CH2Cl2, 25 deg C, 15 min, 2.) 25 deg C, overnight; Multistep reaction; |
-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

-
-
177616-22-7
(Z)-7-((1R,2S,3R,5S)-3,5-Dihydroxy-2-hydroxymethyl-cyclopentyl)-hept-5-enoic acid isopropyl ester

-
-
130209-77-7
15-keto-17-phenyl-18,19,20-trinorprostaglandin F2α isopropyl ester

| Conditions | Yield |
|---|---|
| With aluminium trichloride; 3 A molecular sieve; dihydrogen peroxide; N-ethyl-N,N-diisopropylamine; pyridinium chlorochromate; lithium chloride; phenylboronic acid Yield given. Multistep reaction; |
Dimethyl (2-oxo-4-phenylbutyl)phosphonate Chemical Properties
Molecule structure of Phosphonic acid,P-(2-oxo-4-phenylbutyl)-, dimethyl ester (CAS NO.41162-19-0):
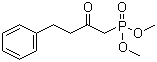
IUPAC Name: 1-Dimethoxyphosphoryl-4-phenylbutan-2-one
Molecular Weight: 256.234741 [g/mol]
Molecular Formula: C12H17O4P
Index of Refraction: 1.494
Molar Refractivity: 64.72 cm3
Molar Volume: 222.2 cm3
Surface Tension: 40.1 dyne/cm
Density: 1.152 g/cm3
Melting Point: 120-122 °C (0.5 mmHg)
Flash Point: 186.8 °C
Enthalpy of Vaporization: 60.87 kJ/mol
Boiling Point: 362.7 °C at 760 mmHg
Vapour Pressure: 1.9E-05 mmHg at 25 °C
XLogP3-AA: 1.1
H-Bond Acceptor: 4
Rotatable Bond Count: 7
Tautomer Count: 3
Exact Mass: 256.086446
MonoIsotopic Mass: 256.086446
Topological Polar Surface Area: 52.6
Heavy Atom Count: 17
Canonical SMILES: COP(=O)(CC(=O)CCC1=CC=CC=C1)OC
InChI: InChI=1S/C12H17O4P/c1-15-17(14,16-2)10-12(13)9-8-11-6-4-3-5-7-11/h3-7H,8-10H2,1-2H3
InChIKey: ONYIBVIIOCEBIV-UHFFFAOYSA-N
Product Categories of Phosphonic acid,P-(2-oxo-4-phenylbutyl)-, dimethyl ester (CAS NO.41162-19-0): Phenyls & Phenyl-Het; Phenyls & Phenyl-Het
Dimethyl (2-oxo-4-phenylbutyl)phosphonate Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 24/25
S24/25:Avoid contact with skin and eyes.
Dimethyl (2-oxo-4-phenylbutyl)phosphonate Specification
Phosphonic acid,P-(2-oxo-4-phenylbutyl)-, dimethyl ester (CAS NO.41162-19-0) is also called Dimethyl(2-oxo-4-phenylbutyl)phosphonate .
Related Products
- Dimethyl ( )-2,3-O-Isopropylidene-D-tartrate
- Dimethyl ((1-methyl-5-nitro-1H-imidazol-2-yl)methylene)propanedioate
- Dimethyl (2-oxo-3,3-difluoroheptyl)phosphonate
- Dimethyl (2-oxo-4-phenylbutyl)phosphonate
- Dimethyl (2-oxoheptyl)phosphonate
- Dimethyl (2S, 2'S)-1, 1'-((2S, 2'S)-2, 2'-(4, 4'-(biphenyl-4, 4'-diyl)bis(1H-imidazole-4, 2-diyl))bis(pyrrolidine-2, 1-diyl))bis(3-methyl-1-oxobutane-2, 1-diyl)dicarbamate
- Dimethyl (3-phenoxy-2-oxopropyl)phosphonate
- Dimethyl (R)-(+)-methylsuccinate
- Dimethyl (S)-(-)-methylsuccinate
- Dimethyl (S)-3-hydroxy-L-aspartate
- 4116-38-5
- 4116-41-0
- 4116-43-2
- 4117-09-3
- 41171-14-6
- 4117-14-0
- 41172-57-0
- 41172-79-6
- 41175-50-2
- 4118-16-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View