-
Name
FLUOROACETYL CHLORIDE
- EINECS 206-623-6
- CAS No. 359-06-8
- Article Data19
- CAS DataBase
- Density 1.278 g/cm3
- Solubility
- Melting Point
- Formula C2H2ClFO
- Boiling Point 49.766 °C at 760 mmHg
- Molecular Weight 96.4887
- Flash Point -16.1 °C
- Transport Information UN 2922
- Appearance Liquid
- Safety 3/7-9-38-45-53
- Risk Codes 14-26-35
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms Acetyl chloride, fluoro-;2-Fluoroacetyl chloride;Chlorid kyseliny fluoroctove;BRN 1739051;HSDB 6327;TL 670;
- PSA 17.07000
- LogP 0.72130
Fluoroacetyl chloride Consensus Reports
EPA Extremely Hazardous Substances List. Reported in EPA TSCA Inventory.
Fluoroacetyl chloride Specification
The Fluoroacetyl chloride with CAS registry number of 359-06-8 is also known as Acetyl chloride,2-fluoro-. The IUPAC name is 2-Fluoroacetyl chloride. In addition, the formula is C2H2ClFO and the molecular weight is 96.49.
Physical properties about Fluoroacetyl chloride are: (1)ACD/LogP: 0.52; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.52; (4)ACD/LogD (pH 7.4): 0.52; (5)ACD/BCF (pH 5.5): 1.45; (6)ACD/BCF (pH 7.4): 1.45; (7)ACD/KOC (pH 5.5): 45.49; (8)ACD/KOC (pH 7.4): 45.49; (9)#H bond acceptors: 1; (10)#Freely Rotating Bonds: 1; (11)Index of Refraction: 1.352; (12)Molar Refractivity: 16.35 cm3; (13)Molar Volume: 75.5 cm3; (14)Surface Tension: 22.6 dyne/cm; (15)Density: 1.277 g/cm3; (16)Enthalpy of Vaporization: 29.33 kJ/mol; (17)Boiling Point: 49.8 °C at 760 mmHg; (18)Vapour Pressure: 294 mmHg at 25 °C.
Preparation of Fluoroacetyl chloride: it is prepared by reaction of fluoroacetic acid. The reaction needs reagent phosphorus V chloride and the yield is about 66%.

Uses of Fluoroacetyl chloride: it is used to produce 2-fluoro-N-hexyl-acetamide by reaction with hexylamine. The reaction occurs with reagent aq. KOH and solvent diethyl ether at the temperature of 5-15 for 5 hours. The yield is about 87%.
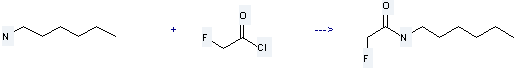
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C(C(=O)Cl)F
2. InChI: InChI=1S/C2H2ClFO/c3-2(5)1-4/h1H2
3. InChIKey: ZBHDTYQJAQDBIH-UHFFFAOYSA-N
Related Products
- Fluoroacetyl chloride
- Fluoroacetyl fluoride
- 359-07-9
- 359-08-0
- 35908-14-6
- 35908-31-7
- 3590-84-9
- 35909-92-3
- 35910-17-9
- 359-10-4
- 35910-59-9
- 359-11-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View