-
Name
PYRITE
- EINECS 235-106-8
- CAS No. 12068-85-8
- Density g/cm3
- Solubility Insoluble in water.
- Melting Point 1171°C
- Formula FeS2
- Boiling Point °Cat760mmHg
- Molecular Weight 119.979
- Flash Point °C
- Transport Information UN3178
- Appearance
- Safety A poison by inhalation and ingestion. The powdered sulfide ignites spontaneously in air and some air-powder mixtures may be explosive. Trace carbon lowers the ignition temperature in air to 228°C and increases the sensitivity of air-dust mixtures. Heats up spontaneously and ignites with combustibles. Incompatible with water. When heated to decomposition or in reaction with acid or acid fumes it emits very toxic fumes of SOx. See also IRON COMPOUNDS, SULFIDES, and HYDROGEN SULFIDE.
- Risk Codes R32; R36/37/38
-
Molecular Structure
- Hazard Symbols Xi
- Synonyms Ferrousdisulfide;Iron disulfide;Iron disulfide (FeS2);iron pyrite;IRON DISULFIDE;IRON SULPHIDE NATURAL POWDER PRS;EISEN(II)-DISULFID, PULVER, SLR, EXTRA PURE;(0698) IRON SULFIDE;ferric sulfide;IRON (III) SULFIDE;ferric oxide yellow;IRON SULFIDE;ferric acid;dodecacarbonyltriiron;Iron disulfide;IRON(II) DISULFIDE, EXTRA PURE, SLR, POWDER;tri-iron dodecacarbonyl;dodecacarbonyl-triangulo-tri-iron;iron dodecarbonyl;
- PSA 64.18000
- LogP 1.29390
Synthetic route
-
-
23550-45-0
disulfur

-
-
557774-20-6
thiozone

-
-
19269-85-3
sulfur

-
-
7439-89-6
iron

-
C
-
12068-85-8
ferric sulfide

-
D
-
142848-03-1
SFeS(1-)

-
E
-
150346-58-0
Fe(S2)2

| Conditions | Yield |
|---|---|
| In solid matrix Nd:YAG laser focused onto metal target (5-10 mJ/pulse), Fe atoms were codeposited with S(x)/Ar mixt. (microwave discharge in Ar seeded with S vapor) onto CsI window cooled to 7 K, annealing to 40 K; IR spect. monitoring, not isolated; |

Iron disulfide Chemical Properties
Molecular Structure of Iron disulfide (CAS NO. 12068-85-8):
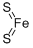
EINECS: 235-106-8
Chemical Name: Iron disulfide
Molecular Formula: FeS2
Molecular Weight: 119.975000 g/mol
H-Bond Donor: 0
H-Bond Acceptor: 2
InChI: InChI=1S/Fe.S2/c;1-2/q+2;-2
InChIKey: NIFIFKQPDTWWGU-UHFFFAOYSA-N
Enthalpy of Vaporization: 29.93 kJ/mol
Boiling Point: 70.7 °C at 760 mmHg
Vapour Pressure: 139 mmHg at 25 °C
Melting Point: 1171°C
Iron disulfide Uses
More than 85 per cent of iron ore is used to make sulfuric acid, followed by sulfur.Pyrite can be used as a combination of copper, lead, zinc, silver, gold, cobalt, nickel, etc.
Iron disulfide Consensus Reports
Reported in EPA TSCA Inventory.
Iron disulfide Safety Profile
Safety Information of Iron disulfide (CAS NO. 12068-85-8):
Risk Statements: 32-36/37/38
R32:Contact with acid liberates very toxic gas
R36/37/38:Irritating to eyes, respiratory system and skin
Safety Statements: 26-36/37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection
RIDADR: UN3178
HazardClass: 4.1
PackingGroup: III
A poison by inhalation and ingestion. The powdered sulfide ignites spontaneously in air and some air-powder mixtures may be explosive. Trace carbon lowers the ignition temperature in air to 228°C and increases the sensitivity of air-dust mixtures. Heats up spontaneously and ignites with combustibles. Incompatible with water. When heated to decomposition or in reaction with acid or acid fumes it emits very toxic fumes of SOx. See also IRON COMPOUNDS, SULFIDES, and HYDROGEN SULFIDE.
Iron disulfide Specification
Iron disulfide with cas registry number of 12068-85-8 is also called for Iron sulfide (FeS2) .
Related Products
- Iron
- Iron alloy, base
- Iron antimonide
- Iron bleomycin
- Iron boride (FeB)
- Iron bromide
- Iron carbide
- Iron chloride sulfate
- Iron citrate tetrahydrate
- Iron difluoride
- 12068-90-5
- 12069-00-0
- 1206905-20-5
- 12069-32-8
- 12069-69-1
- 1206969-99-4
- 1206982-50-4
- 12069-85-1
- 12069-94-2
- 12070-06-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View
