-
Name
Isoprothiolane
- EINECS
- CAS No. 50512-35-1
- Article Data4
- CAS DataBase
- Density 1.228 g/cm3
- Solubility 54 mg l-1 (25 °C)
- Melting Point 54°C
- Formula C12H18O4S2
- Boiling Point 353 °C at 760 mmHg
- Molecular Weight 290.405
- Flash Point 159 °C
- Transport Information
- Appearance
- Safety 61
- Risk Codes 22-51/53
-
Molecular Structure
- Hazard Symbols Xn,N
- Synonyms Isoprothiolane [BSI:ISO];Propanedioic acid, 1,3-dithiolan-2-ylidene-, bis(1-methylethyl) ester;1,3-Dithiolan-2-ylidenepropanedioic acid, bis(1-methylethyl) ester;Fujione;Fudiolan;Di-isopropyl 1,3-dithiolane-2-ylidenemalonate;Bis(1-methylethyl) 1,3-dithiolan-2-ylidenepropanedioate (9CI);
- PSA 103.20000
- LogP 2.58120
Synthetic route
-
-
147848-85-9
3-{2-[2-(2,2-Bis-isopropoxycarbonyl-1-methoxy-vinylsulfanyl)-ethyldisulfanyl]-ethylsulfanyl}-2-isopropoxycarbonyl-3-methoxy-acrylic acid isopropyl ester

-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In tetrahydrofuran; methanol 1.) 3 d, room temperature, 2.) 2 h, reflux; | 64% |
-
-
147848-87-1
2-[(2-Ethyldisulfanyl-ethylsulfanyl)-ethylsulfanyl-methylene]-malonic acid diisopropyl ester

-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol; water Ambient temperature; | 47% |
-
-
67-56-1
methanol

-
-
52303-69-2, 108114-60-9, 108114-61-0
Diisopropyl 1,3-dithiolan-2-ylidenemalonate 1-oxide

-
A
-
50512-35-1
isoprothiolane

-
C
-
147848-85-9
3-{2-[2-(2,2-Bis-isopropoxycarbonyl-1-methoxy-vinylsulfanyl)-ethyldisulfanyl]-ethylsulfanyl}-2-isopropoxycarbonyl-3-methoxy-acrylic acid isopropyl ester

| Conditions | Yield |
|---|---|
| With sodium carbonate for 0.5h; Ambient temperature; | A 17% B 31% C 40% |

-
-
147848-84-8
C26H42O11S4

-
A
-
50512-35-1
isoprothiolane

-
C
-
147848-85-9
3-{2-[2-(2,2-Bis-isopropoxycarbonyl-1-methoxy-vinylsulfanyl)-ethyldisulfanyl]-ethylsulfanyl}-2-isopropoxycarbonyl-3-methoxy-acrylic acid isopropyl ester

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol; water for 12h; Ambient temperature; | A 5% B 27% C 28% |

-
-
18598-63-5
L-cysteine methyl ester hydrochloride

-
-
52303-69-2, 108114-60-9, 108114-61-0
Diisopropyl 1,3-dithiolan-2-ylidenemalonate 1-oxide

-
A
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| With pyridine; acetic anhydride; triethylamine 1.) aq. MeOH, 1 h, room temperature, 2.) 23 h, room temperature; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
52303-69-2, 108114-60-9, 108114-61-0
Diisopropyl 1,3-dithiolan-2-ylidenemalonate 1-oxide

-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 77 percent / methanol / 12 h / Ambient temperature 2: 47 percent / Na2CO3 / methanol; H2O / Ambient temperature View Scheme |
-
-
147848-84-8
C26H42O11S4

-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 28 percent / Na2CO3 / methanol; H2O / 12 h / Ambient temperature 2: 64 percent / NaBH4 / methanol; tetrahydrofuran / 1.) 3 d, room temperature, 2.) 2 h, reflux View Scheme |
-
-
50512-35-1
isoprothiolane

-
-
106262-50-4
2-[1,3]Dithiolan-2-yl-malonic acid diisopropyl ester

| Conditions | Yield |
|---|---|
| With magnesium In methanol at 10℃; for 2.5h; | 93% |
-
-
50512-35-1
isoprothiolane

-
-
52322-05-1
1,3-dithiolan-2-ylidenemalonic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide; water In isopropyl alcohol at 30 - 40℃; for 3h; | 85% |
-
-
50512-35-1
isoprothiolane

-
-
133188-15-5, 147848-83-7
anti-Diisopropyl 1,3-dithiolan-2-ylidenemalonate 1,3-dioxide

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane for 1h; Ambient temperature; | 70% |
-
-
50512-35-1
isoprothiolane

-
-
52303-69-2, 108114-60-9, 108114-61-0
Diisopropyl 1,3-dithiolan-2-ylidenemalonate 1-oxide

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane for 2h; Ambient temperature; | 65% |
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane for 12h; Ambient temperature; | 65% |
-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; water at 25 - 45℃; | 55% |
| With potassium hydroxide | |
| With potassium hydroxide |
-
-
67-56-1
methanol

-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid 1.) CH2Cl2, 12 h, room temperature, 2.) 3 d; Yield given. Multistep reaction; |
-
-
50512-35-1
isoprothiolane

-
A
-
72629-81-3
2-(1,3-dithiolan-2-ylidene)acetic acid

-
B
-
144-62-7
oxalic acid

-
C
-
72629-83-5
3,5-bis-1,2,4-trithiolane

-
D
-
52322-05-1
1,3-dithiolan-2-ylidenemalonic acid

| Conditions | Yield |
|---|---|
| With silica gel for 2.5h; Product distribution; Irradiation; effects of surface and air on pesticide photolysis, different time; different λ; furter pesticides; |

-
-
50512-35-1
isoprothiolane

-
-
109480-62-8
2,2-dimethyl-5-5(2,5-dithiacyclopentylidene)-1,3-dioxane-4,6-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / sodium hydroxide, water / propan-2-ol / 3 h / 30 - 40 °C 2: 1.) conc. sulfuric acid, acetic anhydride / 1.) 10 min, 2.) RT, 8 h View Scheme |
-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / sodium hydroxide, water / propan-2-ol / 3 h / 30 - 40 °C 2: 1.) conc. sulfuric acid, acetic anhydride / 1.) 10 min, 2.) RT, 8 h View Scheme |
-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / sodium hydroxide, water / propan-2-ol / 3 h / 30 - 40 °C 2: 1.) conc. sulfuric acid, acetic anhydride / 1.) 10 min, 2.) RT, 8 h View Scheme |
-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / sodium hydroxide, water / propan-2-ol / 3 h / 30 - 40 °C 2: 1.) conc. sulfuric acid, acetic anhydride / 1.) 10 min, 2.) RT, 8 h View Scheme |
-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / sodium hydroxide, water / propan-2-ol / 3 h / 30 - 40 °C 2: 1.) conc. sulfuric acid, acetic anhydride / 1.) 10 min, 2.) RT, 8 h View Scheme |
-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / sodium hydroxide, water / propan-2-ol / 3 h / 30 - 40 °C 2: 1.) conc. sulfuric acid, acetic anhydride / 1.) 10 min, 2.) RT, 8 h View Scheme |
-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / sodium hydroxide, water / propan-2-ol / 3 h / 30 - 40 °C 2: 1.) conc. sulfuric acid, acetic anhydride / 1.) 10 min, 2.) RT, 8 h View Scheme |
-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / sodium hydroxide, water / propan-2-ol / 3 h / 30 - 40 °C 2: 1.) conc. sulfuric acid, acetic anhydride / 1.) 10 min, 2.) RT, 8 h View Scheme |
-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 65 percent / m-chloroperbenzoic acid (MCPBA) / CH2Cl2 / 12 h / Ambient temperature 2: m-chloroperbenzoic acid (MCPBA) / CH2Cl2 / 12 h / Ambient temperature View Scheme |
-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / sodium hydroxide, water / propan-2-ol / 3 h / 30 - 40 °C 2: 1.) conc. sulfuric acid, acetic anhydride / 1.) 10 min, 2.) RT, 8 h View Scheme |
-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / sodium hydroxide, water / propan-2-ol / 3 h / 30 - 40 °C 2: 1.) conc. sulfuric acid, acetic anhydride / 1.) 10 min, 2.) RT, 8 h View Scheme |
-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 65 percent / m-chloroperbenzoic acid (MCPBA) / CH2Cl2 / 12 h / Ambient temperature 2: 2.) Ac2O, pyridine, NEt3 / 1.) aq. MeOH, 1 h, room temperature, 2.) 23 h, room temperature View Scheme |
-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / sodium hydroxide, water / propan-2-ol / 3 h / 30 - 40 °C 2: 1.) conc. sulfuric acid, acetic anhydride / 1.) 10 min, 2.) RT, 8 h View Scheme |
-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 65 percent / m-chloroperbenzoic acid (MCPBA) / CH2Cl2 / 12 h / Ambient temperature 2: 1.) metachloroperbenzoic acid (MCPBA) / 1.) CH2Cl2, 12 h, room temperature, 2.) MeOH, 2 d, room temperature View Scheme |
-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / sodium hydroxide, water / propan-2-ol / 3 h / 30 - 40 °C 2: 1.) conc. sulfuric acid, acetic anhydride / 1.) 10 min, 2.) RT, 8 h View Scheme |
-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / sodium hydroxide, water / propan-2-ol / 3 h / 30 - 40 °C 2: 1.) conc. sulfuric acid, acetic anhydride / 1.) 10 min, 2.) RT, 8 h View Scheme |
-
-
50512-35-1
isoprothiolane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 65 percent / m-chloroperbenzoic acid (MCPBA) / CH2Cl2 / 12 h / Ambient temperature 2: 94 percent / methanol / Ambient temperature View Scheme |
Isoprothiolane Chemical Properties
Molecular structure of Isoprothiolane (CAS NO.50512-35-1) is:
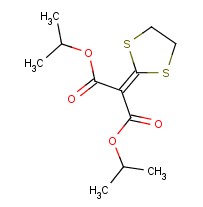
Product Name: Isoprothiolane
CAS Registry Number: 50512-35-1
IUPAC Name: dipropan-2-yl 2-(1,3-dithiolan-2-ylidene)propanedioate
Molecular Weight: 290.39892 [g/mol]
Molecular Formula: C12H18O4S2
XLogP3: 3.3
H-Bond Donor: 0
H-Bond Acceptor: 4
Surface Tension: 45.2 dyne/cm
Density: 1.228 g/cm3
Flash Point: 159 °C
Enthalpy of Vaporization: 59.78 kJ/mol
Boiling Point: 353 °C at 760 mmHg
Vapour Pressure: 3.7E-05 mmHg at 25°C
Product Categories: Fungicide
Isoprothiolane Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| chicken | LD50 | oral | 3860mg/kg (3860mg/kg) | Japan Pesticide Information. Vol. (27), Pg. 20, 1976. | |
| frog | LD50 | oral | 75mg/kg (75mg/kg) | Japan Pesticide Information. Vol. (27), Pg. 20, 1976. | |
| guinea pig | LD50 | oral | 1020mg/kg (1020mg/kg) | Japan Pesticide Information. Vol. (27), Pg. 20, 1976. | |
| mouse | LD50 | intraperitoneal | 440mg/kg (440mg/kg) | Japan Pesticide Information. Vol. (27), Pg. 20, 1976. | |
| mouse | LD50 | oral | 1340mg/kg (1340mg/kg) | Pesticide Manual. Vol. 9, Pg. 506, 1991. | |
| mouse | LD50 | skin | > 10250mg/kg (10250mg/kg) | Japan Pesticide Information. Vol. (27), Pg. 20, 1976. | |
| mouse | LD50 | subcutaneous | > 5gm/kg (5000mg/kg) | Japan Pesticide Information. Vol. (27), Pg. 20, 1976. | |
| rabbit | LD50 | oral | 2320mg/kg (2320mg/kg) | Japan Pesticide Information. Vol. (27), Pg. 20, 1976. | |
| rat | LD50 | intraperitoneal | 480mg/kg (480mg/kg) | Japan Pesticide Information. Vol. (27), Pg. 20, 1976. | |
| rat | LD50 | oral | 1190mg/kg (1190mg/kg) | Farm Chemicals Handbook. Vol. -, Pg. C174, 1991. | |
| rat | LD50 | skin | 10250mg/kg (10250mg/kg) | Japan Pesticide Information. Vol. (40), Pg. 32, 1982. | |
| rat | LD50 | subcutaneous | > 5gm/kg (5000mg/kg) | Japan Pesticide Information. Vol. (27), Pg. 20, 1976. |
Isoprothiolane Specification
Isoprothiolane , its cas register number is 50512-35-1. It also can be called Isoprothiolane [BSI:ISO] ; Bis(1-methylethyl) 1,3-dithiolan-2-ylidenepropanedioate (9CI) ; Di-isopropyl 1,3-dithiolane-2-ylidenemalonate ; Fudiolan ; Fujione ; 1,3-Dithiolan-2-ylidenepropanedioic acid, bis(1-methylethyl) ester ; Propanedioic acid, 1,3-dithiolan-2-ylidene-, bis(1-methylethyl) ester .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View