-
Name
Isoquinolin-6-ol
- EINECS 231-612-8
- CAS No. 7651-82-3
- Article Data13
- CAS DataBase
- Density 1.26 g/cm3
- Solubility
- Melting Point 220ºC
- Formula C9H7 N O
- Boiling Point 332.1 °C at 760 mmHg
- Molecular Weight 145.161
- Flash Point 154.6 °C
- Transport Information
- Appearance white to light yellow crystal powder
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols Xi
- Synonyms 6-Hydroxyisoquinoline
- PSA 33.12000
- LogP 1.94040
Synthetic route

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride; (R)-(+)-(3,5-dioxa-4-phosphacyclohepta[2,1-a;3,4-a']dinaphthalen-4-yl)-5H-dibenz[b,f]azepine; 3,5-dichlorobenzoic acid at 25℃; for 10h; Inert atmosphere; | A 93% B 99% |

-
-
52986-70-6
6-methoxyisoquinoline

-
-
7651-82-3
isoquinolin-6-ol

| Conditions | Yield |
|---|---|
| With aqueous HBr In water | 82% |
| With pyridine hydrochloride at 160℃; | 27% |
| Stage #1: 6-methoxyisoquinoline With pyridine hydrochloride at 160℃; Stage #2: With water; ammonium hydroxide at 20℃; pH=10 - 11; | 27% |
-
-
34784-05-9
6-bromo-isoquinoline

-
-
7651-82-3
isoquinolin-6-ol

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); potassium hydroxide; tert-butyl XPhos In 1,4-dioxane; water at 100℃; for 1.5h; | 75% |

-
-
7651-82-3
isoquinolin-6-ol

| Conditions | Yield |
|---|---|
| With sodiumsulfide nonahydrate In ethanol at 40℃; for 20h; |

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride; (R)-(+)-(3,5-dioxa-4-phosphacyclohepta[2,1-a;3,4-a']dinaphthalen-4-yl)-5H-dibenz[b,f]azepine; 3,5-dichlorobenzoic acid at 25℃; Inert atmosphere; |


| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride; (R)-(+)-(3,5-dioxa-4-phosphacyclohepta[2,1-a;3,4-a']dinaphthalen-4-yl)-5H-dibenz[b,f]azepine; 3,5-dichlorobenzoic acid at 25℃; Inert atmosphere; |

-
-
7651-82-3
isoquinolin-6-ol

-
-
918488-43-4
5-iodoisoquinolin-6-ol

| Conditions | Yield |
|---|---|
| Stage #1: isoquinolin-6-ol With trifluorormethanesulfonic acid; [bis(pyridine)iodine]+ tetrafluoroborate In dichloromethane at 0 - 20℃; for 3h; Stage #2: With sodium hydrogencarbonate In water | 97% |

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 21h; Mitsunobu Displacement; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With sodium hydride In 1,4-dioxane; mineral oil at 100℃; for 8h; | 95% |
-
-
7651-82-3
isoquinolin-6-ol

-
-
918488-42-3
5-bromoisoquinoline-6-ol

| Conditions | Yield |
|---|---|
| With flavin reductase enzyme from E. Coli; radicicol halogenase from Chaetomium chiversii D456E/T501S mutant; β-nicotinamide adenine dinucleotide reduced; riboflavin adenine dinucleotide; potassium bromide In aq. phosphate buffer; ethanol at 30℃; for 2h; pH=7.4; Enzymatic reaction; regioselective reaction; | 92% |
| Stage #1: isoquinolin-6-ol With bromine In chloroform at 20℃; for 2h; Stage #2: With sodium hydrogencarbonate In water | 88% |
| Stage #1: isoquinolin-6-ol With bromine In chloroform at 20℃; for 2h; Stage #2: With water; sodium hydrogencarbonate pH=8; | 88% |
-
-
7651-82-3
isoquinolin-6-ol

-
-
918488-41-2
5-chloro-6-hydroxyisoquinoline

| Conditions | Yield |
|---|---|
| Stage #1: isoquinolin-6-ol With sulfuryl dichloride In diethyl ether; dichloromethane at 20℃; for 5h; Stage #2: With sodium hydrogencarbonate In water | 89% |
| Stage #1: isoquinolin-6-ol With sulfuryl dichloride In dichloromethane at 20℃; for 5h; Stage #2: With water; sodium hydrogencarbonate | 89% |
| With Escherichia coli BL21-CodonPlus (DE3)-RIL/pJZ54 Enzymatic reaction; | 11.4 mg |
| With flavin reductase enzyme from E. Coli; radicicol halogenase from Chaetomium chiversii D456E/T501S mutant; β-nicotinamide adenine dinucleotide reduced; riboflavin adenine dinucleotide; magnesium chloride In aq. phosphate buffer; ethanol at 30℃; for 2h; pH=7.4; Kinetics; Catalytic behavior; Reagent/catalyst; Enzymatic reaction; regioselective reaction; |

| Conditions | Yield |
|---|---|
| With trifluoroacetic anhydride In dichloromethane at 20℃; for 1h; Inert atmosphere; Glovebox; Sealed tube; | 84% |

| Conditions | Yield |
|---|---|
| With (S)-(+)-N-(3,5-dioxa-4-phosphacyclohepta-[2,1-a;3,4-a′]dinaphthalen-4-yl)dibenz[b,f]azepine; bis(1,5-cyclooctadiene)diiridium(I) dichloride; 3,5-dichlorobenzoic acid In methanol at 25℃; for 0.1h; Inert atmosphere; enantioselective reaction; | 82% |

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 16h; Mitsunobu Displacement; Inert atmosphere; | 80% |
-
-
7651-82-3
isoquinolin-6-ol

-
-
1073277-35-6
(+/-)-1-(4'-fluorophenyl)prop-2-en-1-yl tert-butyl carbonate

| Conditions | Yield |
|---|---|
| With (S)-(+)-N-(3,5-dioxa-4-phosphacyclohepta-[2,1-a;3,4-a′]dinaphthalen-4-yl)dibenz[b,f]azepine; bis(1,5-cyclooctadiene)diiridium(I) dichloride In methanol at 25℃; for 0.166667h; Inert atmosphere; enantioselective reaction; | 80% |
-
-
7651-82-3
isoquinolin-6-ol

-
-
444575-79-5
carbonic acid 1,1-dimethylethyl 1-phenyl-2-propenyl ester

| Conditions | Yield |
|---|---|
| With (S)-(+)-N-(3,5-dioxa-4-phosphacyclohepta-[2,1-a;3,4-a′]dinaphthalen-4-yl)dibenz[b,f]azepine; bis(1,5-cyclooctadiene)diiridium(I) dichloride In methanol at 25℃; for 0.1h; Inert atmosphere; enantioselective reaction; | 80% |

| Conditions | Yield |
|---|---|
| With (S)-(+)-N-(3,5-dioxa-4-phosphacyclohepta-[2,1-a;3,4-a′]dinaphthalen-4-yl)dibenz[b,f]azepine; bis(1,5-cyclooctadiene)diiridium(I) dichloride; 3,5-dichlorobenzoic acid In methanol at 25℃; for 10h; Inert atmosphere; enantioselective reaction; | 78% |
-
-
7651-82-3
isoquinolin-6-ol

-
-
444575-79-5
carbonic acid 1,1-dimethylethyl 1-phenyl-2-propenyl ester

| Conditions | Yield |
|---|---|
| With (S)-(+)-N-(3,5-dioxa-4-phosphacyclohepta-[2,1-a;3,4-a′]dinaphthalen-4-yl)dibenz[b,f]azepine; bis(1,5-cyclooctadiene)diiridium(I) dichloride; 3,5-dichlorobenzoic acid In methanol at 25℃; for 10h; Inert atmosphere; enantioselective reaction; | 78% |
-
-
7651-82-3
isoquinolin-6-ol

-
-
1073277-35-6
(+/-)-1-(4'-fluorophenyl)prop-2-en-1-yl tert-butyl carbonate

| Conditions | Yield |
|---|---|
| With (S)-(+)-N-(3,5-dioxa-4-phosphacyclohepta-[2,1-a;3,4-a′]dinaphthalen-4-yl)dibenz[b,f]azepine; bis(1,5-cyclooctadiene)diiridium(I) dichloride; 3,5-dichlorobenzoic acid In methanol at 25℃; for 10h; Inert atmosphere; enantioselective reaction; | 77% |

| Conditions | Yield |
|---|---|
| With (S)-(+)-N-(3,5-dioxa-4-phosphacyclohepta-[2,1-a;3,4-a′]dinaphthalen-4-yl)dibenz[b,f]azepine; bis(1,5-cyclooctadiene)diiridium(I) dichloride; dibenzenesulfonamide In methanol at 25℃; for 11h; Inert atmosphere; enantioselective reaction; | 77% |
-
-
7651-82-3
isoquinolin-6-ol

-
-
23426-63-3
2-bromo-2-methylpropionic acid methyl ester

-
-
945860-77-5
methyl 2-(isoquinolin-6-yloxy)-2-methylpropanoate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; for 16h; | 74% |
-
-
7651-82-3
isoquinolin-6-ol

-
-
107-14-2
chloroacetonitrile

-
-
1383730-50-4
2-(cyanomethyl)-6-hydroxyisoquinolinium chloride

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 4h; Reflux; | 74% |

| Conditions | Yield |
|---|---|
| With (S)-(+)-N-(3,5-dioxa-4-phosphacyclohepta-[2,1-a;3,4-a′]dinaphthalen-4-yl)dibenz[b,f]azepine; bis(1,5-cyclooctadiene)diiridium(I) dichloride; 3,5-dichlorobenzoic acid In methanol at 25℃; for 10h; Inert atmosphere; enantioselective reaction; | 74% |

| Conditions | Yield |
|---|---|
| With (S)-(+)-N-(3,5-dioxa-4-phosphacyclohepta-[2,1-a;3,4-a′]dinaphthalen-4-yl)dibenz[b,f]azepine; bis(1,5-cyclooctadiene)diiridium(I) dichloride In methanol at 25℃; for 0.133333h; Inert atmosphere; enantioselective reaction; | 73% |

| Conditions | Yield |
|---|---|
| With (S)-(+)-N-(3,5-dioxa-4-phosphacyclohepta-[2,1-a;3,4-a′]dinaphthalen-4-yl)dibenz[b,f]azepine; bis(1,5-cyclooctadiene)diiridium(I) dichloride; 3,5-dichlorobenzoic acid In methanol at 25℃; for 10h; Inert atmosphere; enantioselective reaction; | 72% |

| Conditions | Yield |
|---|---|
| With N,N'-1,3-bis(2,6-diisopropylphenyl)-4,5-dihydro-2,2-difluoroimidazolidine; cesium fluoride In 1,4-dioxane at 23 - 110℃; for 24.5h; Inert atmosphere; | 71% |
-
-
7651-82-3
isoquinolin-6-ol

-
-
1073277-21-0
(+/-)-1-(4'-bromophenyl)prop-2-en-1-yl tert-butyl carbonate

| Conditions | Yield |
|---|---|
| With (S)-(+)-N-(3,5-dioxa-4-phosphacyclohepta-[2,1-a;3,4-a′]dinaphthalen-4-yl)dibenz[b,f]azepine; bis(1,5-cyclooctadiene)diiridium(I) dichloride; 3,5-dichlorobenzoic acid In methanol at 25℃; for 10h; Inert atmosphere; enantioselective reaction; | 71% |

| Conditions | Yield |
|---|---|
| With (S)-(+)-N-(3,5-dioxa-4-phosphacyclohepta-[2,1-a;3,4-a′]dinaphthalen-4-yl)dibenz[b,f]azepine; bis(1,5-cyclooctadiene)diiridium(I) dichloride; 3,5-dichlorobenzoic acid In methanol at 25℃; for 10h; Inert atmosphere; enantioselective reaction; | 71% |

| Conditions | Yield |
|---|---|
| With (S)-(+)-N-(3,5-dioxa-4-phosphacyclohepta-[2,1-a;3,4-a′]dinaphthalen-4-yl)dibenz[b,f]azepine; bis(1,5-cyclooctadiene)diiridium(I) dichloride; 3,5-dichlorobenzoic acid In methanol at 25℃; for 10h; Inert atmosphere; enantioselective reaction; | 71% |

| Conditions | Yield |
|---|---|
| With (S)-(+)-N-(3,5-dioxa-4-phosphacyclohepta-[2,1-a;3,4-a′]dinaphthalen-4-yl)dibenz[b,f]azepine; bis(1,5-cyclooctadiene)diiridium(I) dichloride; dibenzenesulfonamide In methanol at 25℃; for 9h; Inert atmosphere; enantioselective reaction; | 68% |

| Conditions | Yield |
|---|---|
| In water | 67% |
-
-
7651-82-3
isoquinolin-6-ol

-
-
1073277-21-0
(+/-)-1-(4'-bromophenyl)prop-2-en-1-yl tert-butyl carbonate

| Conditions | Yield |
|---|---|
| With (S)-(+)-N-(3,5-dioxa-4-phosphacyclohepta-[2,1-a;3,4-a′]dinaphthalen-4-yl)dibenz[b,f]azepine; bis(1,5-cyclooctadiene)diiridium(I) dichloride In methanol at 25℃; for 0.166667h; Inert atmosphere; enantioselective reaction; | 66% |

| Conditions | Yield |
|---|---|
| With (S)-(+)-N-(3,5-dioxa-4-phosphacyclohepta-[2,1-a;3,4-a′]dinaphthalen-4-yl)dibenz[b,f]azepine; bis(1,5-cyclooctadiene)diiridium(I) dichloride In methanol at 25℃; for 0.166667h; Inert atmosphere; enantioselective reaction; | 65% |
Isoquinolin-6-ol Chemical Properties
Molecular structure of Isoquinolin-6-ol (CAS NO.7651-82-3) is:
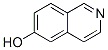
Product Name: Isoquinolin-6-ol
CAS Registry Number: 7651-82-3
IUPAC Name: 2H-isoquinolin-6-one
Molecular Weight: 145.15798 [g/mol]
Molecular Formula: C9H7NO
XLogP3-AA: 0.5
H-Bond Donor: 1
H-Bond Acceptor: 2
EINECS: 231-612-8
Molar Volume: 115.203 cm3
Surface Tension: 59.731 dyne/cm
Density: 1.26 g/cm3
Flash Point: 154.633 °C
Enthalpy of Vaporization: 59.761 kJ/mol
Boiling Point: 332.074 °C at 760 mmHg
Product Categories: blocks ;Heterocycles ;Quinolines ;Heterocyclic Series ;Quinoline&Isoquinoline ;Building Blocks ;Isoquinoline
Isoquinolin-6-ol Safety Profile
Hazard Codes:  Xi
Xi
Hazard Note: Irritant
Isoquinolin-6-ol Specification
Isoquinolin-6-ol , its cas register number is 7651-82-3. It also can be called EINECS 231-612-8 .It is a white to light yellow crystal powder.
Related Products
- Isoquinolin-6-ol
- 7651-83-4
- 7651-99-2
- 765211-09-4
- 7652-29-1
- 76523-24-5
- 76523-40-5
- 7652-46-2
- 765256-39-1
- 765263-36-3
- 7652-64-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View