-
Name
Itopride hydrochloride
- EINECS 602-905-1
- CAS No. 122892-31-3
- Article Data10
- CAS DataBase
- Density
- Solubility
- Melting Point 194-195 °C
- Formula C20H26N2O4.HCl
- Boiling Point 510.1 °C at 760 mmHg
- Molecular Weight 394.898
- Flash Point 262.3 °C
- Transport Information
- Appearance white or pale brownish crystal
- Safety 61
- Risk Codes 50
-
Molecular Structure
- Hazard Symbols N
- Synonyms Benzamide,N-[[4-[2-(dimethylamino)ethoxy]phenyl]methyl]-3,4-dimethoxy-, monohydrochloride(9CI);Ganaton;HSR 803;
- PSA 60.03000
- LogP 3.76710
Synthetic route
-
-
122898-67-3
itopride

-
-
122892-31-3
N-<4-<2-(dimethylamino)ethoxy>benzyl>-3,4-dimethoxybenzamide hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water; iso-butanol at 50 - 55℃; for 0.5h; pH=2.0; | 93.7% |
| With hydrogenchloride In ethanol at 0℃; | 93% |
| With hydrogenchloride In ethanol | 90% |
| With hydrogenchloride In isopropyl alcohol at 25 - 30℃; for 0.5h; pH=1 - 2; | 79% |
| With hydrogenchloride In isopropyl alcohol at 25 - 30℃; for 0.5h; pH=1 - 2; | 79% |
-
-
2024-83-1
veratronitrile

-
-
131028-54-1
(4-(2-(dimethylamino)ethoxy)phenyl)methanol

-
-
122898-67-3
itopride

-
-
122892-31-3
N-<4-<2-(dimethylamino)ethoxy>benzyl>-3,4-dimethoxybenzamide hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: veratronitrile; (4-(2-(dimethylamino)ethoxy)phenyl)methanol With trifluorormethanesulfonic acid In toluene at 80℃; for 8h; Stage #2: itopride With hydrogenchloride In ethanol at 0℃; Reagent/catalyst; | 93% |

-
-
943518-63-6
N-(4-hydroxybenzyl)-3,4-dimethoxybenzamide

-
-
107-99-3
2-(dimethylamino)ethyl chloride

-
-
122892-31-3
N-<4-<2-(dimethylamino)ethoxy>benzyl>-3,4-dimethoxybenzamide hydrochloride

| Conditions | Yield |
|---|---|
| With potassium iodide In N,N-dimethyl-formamide at 130 - 135℃; Temperature; | 87% |
-
-
108-01-0
2-(N,N-dimethylamino)ethanol

-
-
122892-31-3
N-<4-<2-(dimethylamino)ethoxy>benzyl>-3,4-dimethoxybenzamide hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: 2-(N,N-dimethylamino)ethanol With sodium hydroxide In toluene for 0.5h; Reflux; Stage #2: 4-((3,4-dimethoxybenzamido)methyl)phenyl methanesulfonate In toluene for 1.5h; Reflux; Stage #3: With hydrogenchloride In dichloromethane; water for 0.5h; | 80% |
-
-
767-00-0
4-cyanophenol

-
-
122892-31-3
N-<4-<2-(dimethylamino)ethoxy>benzyl>-3,4-dimethoxybenzamide hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: triethylamine / dichloromethane / 0.5 h 1.2: 1 h 2.1: nickel dichloride; sodium tetrahydroborate / ethanol / 0.5 h / 20 - 25 °C 3.1: triethylamine / methanol / 2 h 4.1: sodium hydroxide / toluene / 0.5 h / Reflux 4.2: 1.5 h / Reflux 4.3: 0.5 h View Scheme | |
| Multi-step reaction with 4 steps 1.1: triethylamine / dichloromethane / 0.5 h 1.2: 1 h 2.1: nickel dichloride; sodium tetrahydroborate / ethanol / 0.5 h / 20 - 25 °C 3.1: 1-hydroxy-pyrrolidine-2,5-dione / dichloromethane / 0.17 h / -10 - 0 °C 3.2: 2 h / Reflux 3.3: 2 h / Reflux 4.1: sodium hydroxide / toluene / 0.5 h / Reflux 4.2: 1.5 h / Reflux 4.3: 0.5 h View Scheme |
-
-
138373-10-1
4-cyanophenyl mesylate

-
-
122892-31-3
N-<4-<2-(dimethylamino)ethoxy>benzyl>-3,4-dimethoxybenzamide hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: nickel dichloride; sodium tetrahydroborate / ethanol / 0.5 h / 20 - 25 °C 2.1: triethylamine / methanol / 2 h 3.1: sodium hydroxide / toluene / 0.5 h / Reflux 3.2: 1.5 h / Reflux 3.3: 0.5 h View Scheme | |
| Multi-step reaction with 3 steps 1.1: nickel dichloride; sodium tetrahydroborate / ethanol / 0.5 h / 20 - 25 °C 2.1: 1-hydroxy-pyrrolidine-2,5-dione / dichloromethane / 0.17 h / -10 - 0 °C 2.2: 2 h / Reflux 2.3: 2 h / Reflux 3.1: sodium hydroxide / toluene / 0.5 h / Reflux 3.2: 1.5 h / Reflux 3.3: 0.5 h View Scheme |
-
-
1052148-15-8
(4-methanesulfonyloxyphenylmethane)amine

-
-
122892-31-3
N-<4-<2-(dimethylamino)ethoxy>benzyl>-3,4-dimethoxybenzamide hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: triethylamine / methanol / 2 h 2.1: sodium hydroxide / toluene / 0.5 h / Reflux 2.2: 1.5 h / Reflux 2.3: 0.5 h View Scheme |
-
-
108-95-2
phenol

-
-
122892-31-3
N-<4-<2-(dimethylamino)ethoxy>benzyl>-3,4-dimethoxybenzamide hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: potassium hydroxide / ethanol; water / 4 h / 55 - 60 °C 2: hydrogenchloride / water / 2 h / -10 °C 3: ammonium hydroxide / methanol / 6 h / 80 °C / Autoclave 4: N-ethyl-N,N-diisopropylamine / toluene / 2 h / 70 °C / Cooling with ice 5: hydrogenchloride / ethanol View Scheme |
-
-
13468-02-5
dimethyl(2-phenoxyethyl)amine

-
-
122892-31-3
N-<4-<2-(dimethylamino)ethoxy>benzyl>-3,4-dimethoxybenzamide hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: hydrogenchloride / water / 2 h / -10 °C 2: ammonium hydroxide / methanol / 6 h / 80 °C / Autoclave 3: N-ethyl-N,N-diisopropylamine / toluene / 2 h / 70 °C / Cooling with ice 4: hydrogenchloride / ethanol View Scheme |
-
-
131028-55-2
[2-(4-Chloromethyl-phenoxy)-ethyl]-dimethyl-amine

-
-
122892-31-3
N-<4-<2-(dimethylamino)ethoxy>benzyl>-3,4-dimethoxybenzamide hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: ammonium hydroxide / methanol / 6 h / 80 °C / Autoclave 2: N-ethyl-N,N-diisopropylamine / toluene / 2 h / 70 °C / Cooling with ice 3: hydrogenchloride / ethanol View Scheme |
-
-
123-08-0
4-hydroxy-benzaldehyde

-
-
122892-31-3
N-<4-<2-(dimethylamino)ethoxy>benzyl>-3,4-dimethoxybenzamide hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: potassium carbonate; triethylamine / N,N-dimethyl-formamide / 2 h / 80 °C 2.1: methanol; sodium tetrahydroborate / Cooling with ice 3.1: trifluorormethanesulfonic acid / toluene / 8 h / 80 °C 3.2: 0 °C View Scheme |
-
-
106-44-5
p-cresol

-
-
122892-31-3
N-<4-<2-(dimethylamino)ethoxy>benzyl>-3,4-dimethoxybenzamide hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: triethylamine; potassium hydroxide / di-isopropyl ether; N,N-dimethyl-formamide / 2 h / 80 °C 2: N-Bromosuccinimide / chloroform / 5 h / Reflux 3: copper(II) bis(trifluoromethanesulfonate) / 5 h / 100 °C 4: hydrogenchloride / ethanol / 0 °C View Scheme |
-
-
51344-14-0
N,N-dimethyl-2-<4-methylphenoxy>-ethylamine

-
-
122892-31-3
N-<4-<2-(dimethylamino)ethoxy>benzyl>-3,4-dimethoxybenzamide hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: N-Bromosuccinimide / chloroform / 5 h / Reflux 2: copper(II) bis(trifluoromethanesulfonate) / 5 h / 100 °C 3: hydrogenchloride / ethanol / 0 °C View Scheme |
-
-
120-14-9
3,4-dimethoxy-benzaldehyde

-
-
122892-31-3
N-<4-<2-(dimethylamino)ethoxy>benzyl>-3,4-dimethoxybenzamide hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: hydroxylamine hydrochloride / ethanol / Reflux 1.2: 6 h / Reflux 2.1: copper(II) bis(trifluoromethanesulfonate) / 5 h / 100 °C 3.1: hydrogenchloride / ethanol / 0 °C View Scheme |
Itopride hydrochloride Chemical Properties
IUPAC Name: N-[[4-(2-Dimethylaminoethyloxy)phenyl]methyl]-3,4-dimethoxybenzamide hydrochloride
CAS: 122892-31-3
Formula: C20H27ClN2O4
Molecular Weight: 394.89
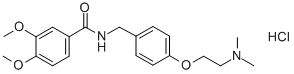
Molecular Structure of N-[[4-(2-Dimethylaminoethyloxy)phenyl]methyl]-3,4-dimethoxybenzamide hydrochloride (122892-31-3 ):
Flash Point: 262.3 °C
Melting Point: 194-195 °C
Boiling Point: 510.1 °C at 760 mmHg
Vapour Pressure: 1.6E-10 mmHg at 25°C
Appearance: Crystalline Solid
Enthalpy of Vaporization: 78.08 kJ/mol
The classification of N-[[4-(2-Dimethylaminoethyloxy)phenyl]methyl]-3,4-dimethoxybenzamide hydrochloride (122892-31-3) are Active Pharmaceutical Ingredients;Intermediates & Fine Chemicals;Pharmaceuticals;Itopride.
Itopride hydrochloride Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | oral | 600mg/kg (600mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 26, Pg. 740, 1995. | |
| mouse | LD50 | intravenous | 164mg/kg (164mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 26, Pg. 740, 1995. | |
| mouse | LD50 | oral | > 2gm/kg (2000mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 26, Pg. 740, 1995. | |
| rat | LD50 | intravenous | 275mg/kg (275mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 26, Pg. 740, 1995. | |
| rat | LD50 | oral | > 2gm/kg (2000mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 26, Pg. 740, 1995. |
Itopride hydrochloride Specification
The chemical synonyms of N-[[4-(2-Dimethylaminoethyloxy)phenyl]methyl]-3,4-dimethoxybenzamide hydrochloride (122892-31-3) are N-((4-(2-(Dimethylamino)ethoxy)phenyl)methyl)-3,4-dimethoxybenzamidemonohydr ; N-((4-(2-(Dimethylamino)ethoxy)phenyl)methyl)-3,4-dimethoxy-benzamidmonohy ; N-((4-(2-(Dimethylamino)ethoxy)phenyl)methyl)-3,4-dimethoxy-benzamidmonohydrochloride ; N-(4-(2-(Dimethylamino)ethoxy)benzyl)-3,4-dimethoxidebenzamidehydrochloride .The product of N-[[4-(2-Dimethylaminoethyloxy)phenyl]methyl]-3,4-dimethoxybenzamide hydrochloride (122892-31-3 ) can enhance Dopamine D2-receptor antagonist with anticholinesterase activity. Gastroprokinetic.
Related Products
- Itopride hydrochloride
- 122894-56-8
- 122894-64-8
- 122894-73-9
- 122898-67-3
- 12289-94-0
- 1229000-10-5
- 1229005-35-9
- 122908-18-3
- 122913-92-2
- 122918-25-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View