-
Name
METHYL PYRROLE-1-CARBOXYLATE
- EINECS 224-281-6
- CAS No. 4277-63-8
- Article Data18
- CAS DataBase
- Density 1.09 g/cm3
- Solubility
- Melting Point 166-167°C
- Formula C6H7NO2
- Boiling Point 182.5 °C at 760 mmHg
- Molecular Weight 125.127
- Flash Point 64.2 °C
- Transport Information UN 3272 3/PG 3
- Appearance clear colorless to very slightly yellow liquid
- Safety 24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Pyrrole-1-carboxylicacid, methyl ester (6CI,7CI,8CI);Methyl 1-pyrrolecarboxylate;Methyl1H-pyrrole-1-carboxylate;N-(Methoxycarbonyl)pyrrole;N-Carbomethoxypyrrole;
- PSA 31.23000
- LogP 1.10260
Synthetic route
-
-
109-97-7
pyrrole

-
-
616-38-6
carbonic acid dimethyl ester

-
A
-
96-54-8
N-Methylpyrrole

-
B
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

| Conditions | Yield |
|---|---|
| tetrabutylammomium bromide at 110℃; atmospheric pressure; | A 4% B 92% |
| tetrabutylammomium bromide at 120℃; for 24h; atmospheric pressure; | A 36% B 51% |
| With tert-butylimino-tri(pyrrolidino)phosphorane at 119.84℃; for 6.5h; Product distribution; Further Variations:; Reagents; Temperatures; reaction time; | A n/a B 68 % Chromat. |
| With 1,4-diaza-bicyclo[2.2.2]octane In N,N-dimethyl-formamide at 90 - 92℃; for 24h; |


| Conditions | Yield |
|---|---|
| With porous poly(ionic liquid) prepared by the anion exchange of poly(bisvinylimidazolium-base disalicylate) and NaCl at 110℃; for 9h; Reagent/catalyst; | 87% |
-
-
109-97-7
pyrrole

-
-
1309041-52-8
methyl 1,8-diazabicyclo[5.4.0]undec-6-ene-8-carboxylate

-
A
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
B
-
6674-22-2
1,8-diazabicyclo[5.4.0]undec-7-ene

| Conditions | Yield |
|---|---|
| In chloroform-d1 at 19.84℃; for 56h; | A 78% B n/a |

-
-
124-41-4
sodium methylate

-
-
107962-24-3
pyrrole-1-carboxylic acid anhydride

-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -20℃; for 0.25h; | 69% |
-
-
66893-74-1
1-methoxycarbonyl-2,5-dimethoxypyrrolidine

-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 4h; Heating; | 62% |
-
-
85684-89-5
1-triphenylmethylpyrrole

-
A
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
B
-
789-24-2
9-Phenylfluorene

-
C
-
5467-21-0
methyl 2,2,2-triphenylacetate

| Conditions | Yield |
|---|---|
| With n-butyllithium In hexane; N,N-dimethyl-formamide for 2h; Ambient temperature; | A 25% B 31% C 60% |


| Conditions | Yield |
|---|---|
| In diethyl ether; hexane for 2h; Heating; | 40% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
85684-89-5
1-triphenylmethylpyrrole

-
-
124-38-9
carbon dioxide

-
A
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
B
-
789-24-2
9-Phenylfluorene

-
C
-
5467-21-0
methyl 2,2,2-triphenylacetate

| Conditions | Yield |
|---|---|
| With n-butyllithium 1.) DMF, room temperature, 2 h; ether, 2.) ether, 0 deg C; Yield given. Multistep reaction. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With potassium hydride In tetrahydrofuran at 0℃; | |
| With sodium hydride |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
18276-53-4
1-(trimethylsilyl)-1H-pyrrole

-
-
124-38-9
carbon dioxide

-
A
-
1193-62-0
methyl Pyrrole-2-carboxylate

-
B
-
2703-17-5
methyl 1H-pyrrole-3-carboxylate

-
C
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
D
-
1757-29-5
1H-pyrrole-2,5-dicarboxylic acid dimethyl ester

| Conditions | Yield |
|---|---|
| Product distribution; other time, other temperature; lithiations of 1-trialkylsilylpyrroles in multistep reactions; | |
| Yield given. Multistep reaction. Yields of byproduct given; |

-
-
186581-53-3, 908094-01-9
diazomethane

-
-
18276-53-4
1-(trimethylsilyl)-1H-pyrrole

-
-
124-38-9
carbon dioxide

-
A
-
1193-62-0
methyl Pyrrole-2-carboxylate

-
B
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
C
-
1757-29-5
1H-pyrrole-2,5-dicarboxylic acid dimethyl ester

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction. Yields of byproduct given; |

-
-
186581-53-3, 908094-01-9
diazomethane

-
-
18276-53-4
1-(trimethylsilyl)-1H-pyrrole

-
-
124-38-9
carbon dioxide

-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction; |

-
-
21972-99-6
pyrrole-1-carboxylic acid

-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 87 percent / 1-<3-(dimethylamino)propyl>-3-ethylcarbodiimide hydrochloride (EDCl*HCl) / CH2Cl2 / 0.25 h / 25 °C 2: 69 percent / tetrahydrofuran / 0.25 h / -20 °C View Scheme |
-
-
56475-80-0
N-(methoxycarbonyl)pyrrolidine

-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 77 percent / anodic oxidation in the tetraethylammonium p-toluenesulfonate 2: 62 percent / TsOH / benzene / 4 h / Heating View Scheme |
-
-
109-97-7
pyrrole

-
-
13509-27-8
methyl phenyl carbonate

-
A
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
B
-
56880-01-4
1-phenoxycarbonyl pyrrole

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene at 119.84℃; for 24h; Product distribution; Further Variations:; Temperatures; reaction time; | A 47 % Chromat. B 12 % Chromat. |

-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
762-42-5
dimethyl acetylenedicarboxylate

-
-
19169-19-8
trimethyl 7-azabicyclo[2.2.1]hepta-2,5-diene-2,3,7-tricarboxylate

| Conditions | Yield |
|---|---|
| With aluminium trichloride In dichloromethane at 40℃; for 1h; | 90% |
-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
1408320-94-4
N-chloro-N-(phenylmethoxy)-N'-(phenylmethyl)urea

| Conditions | Yield |
|---|---|
| With sodium 2,2,3,3-tetrafluoropropan-1-olate In acetonitrile at 0℃; Inert atmosphere; | 90% |
-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
82853-93-8
N,N′-dibenzyloxyurea

| Conditions | Yield |
|---|---|
| With 2,2,3,3-tetrafluoropropanol; [bis(acetoxy)iodo]benzene; sodium 2,2,3,3-tetrafluoropropan-1-olate In acetonitrile at 0℃; for 3.5h; Inert atmosphere; | 86% |
-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
13894-21-8
ethynyl p-tolyl sulfone

-
-
83060-74-6
(1S,4R)-2-(Toluene-4-sulfonyl)-7-aza-bicyclo[2.2.1]hepta-2,5-diene-7-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| at 85℃; for 24h; | 85% |
| at 80 - 85℃; for 24h; | 60% |
| at 80 - 85℃; for 48h; Yield given; |
-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
90388-37-7
N-(2,4-dichloro-6-oxo-2,4-cyclohexadien-1-ylidene)-4-nitrobenzamide

| Conditions | Yield |
|---|---|
| In dichloromethane for 17h; Ambient temperature; | 83% |
-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
13894-21-8
ethynyl p-tolyl sulfone

-
-
83060-74-6
7-methoxycarbonyl-2-p-toluenesulfonyl-7-azanorbornadiene

| Conditions | Yield |
|---|---|
| In dichloromethane under 9000720 Torr; | 81% |
| at 85 - 90℃; | 72% |
| at 85℃; for 26h; | 67% |
-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
815-60-1
2,4-dibromo-3-pentanone

-
A
-
53406-52-3
methyl 2α,4α-dimethyl-3-oxo-8-azabicyclo<3.2.1>oct-6-ene-8-carboxylate

| Conditions | Yield |
|---|---|
| With diethylzinc In hexane; toluene | A 81% B 6% |
| With zinc; 1,1-dibromomethane In acetonitrile at 20℃; for 1h; ultrasonification; Title compound not separated from byproducts; |

-
-
75-77-4
chloro-trimethyl-silane

-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
1199553-52-0
methyl 2,5-bis(trimethylsilyl)-1H-pyrrole-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: methyl 1H-pyrrole-1-carboxylate With 2,2,6,6-tetramethyl-piperidine; n-butyllithium In tetrahydrofuran; hexane at -78℃; for 1.5h; Inert atmosphere; Stage #2: chloro-trimethyl-silane In tetrahydrofuran; hexane at -78 - 20℃; for 2.5h; Inert atmosphere; | 80% |
-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
359779-60-5
4-bromo-1,1,1-trifluorobut-3-yn-2-one

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 20℃; Inert atmosphere; | 78% |
| 76% |
-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
126554-35-6
methyl 2-diazobut-3-enoate

-
-
126554-31-2
Methyl N-(methoxycarbonyl)-8-azabicyclo<3.2.1>octa-2,6-diene-2-carboxylate

| Conditions | Yield |
|---|---|
| With rhodium(II) hexanoate In hexane for 12h; Heating; | 75% |
| rhodium(II) acetate | 21% |
-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
152328-56-8
phenylsulfonyl 6-chloro-3-pyridyl acetylene

-
-
163299-82-9
2-Benzenesulfonyl-3-(6-chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]hepta-2,5-diene-7-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| at 80 - 85℃; for 24h; | 70% |
-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
227454-51-5
1-((bromoethynyl)sulfonyl)-4-methylbenzene

-
-
227454-64-0
N-methoxycarbonyl-7-aza-2-bromo-3-p-tolylsulfonylbicyclo[2.2.1]hepta-2,5-diene

| Conditions | Yield |
|---|---|
| In toluene at 90℃; for 24h; | 70% |
-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
38651-07-9
1,3-dibromo-1-phenylbutan-2-one

| Conditions | Yield |
|---|---|
| With diethylzinc In hexane; toluene | A 5% B 69% |


| Conditions | Yield |
|---|---|
| With tris(4-anisyl)amine; 2-butyl-4,7-bis(1-methyl-1H-pyrrol-2-yl)-2H-benzo[d][1,2,3]triazole In dimethyl sulfoxide at 20℃; for 24h; Irradiation; Schlenk technique; Inert atmosphere; | 69% |
-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
104525-94-2
diethyl (E)-4-diazopent-2-enedioate

-
-
126554-26-5, 126554-39-0
(1S,4R,5R)-8-Aza-bicyclo[3.2.1]octa-2,6-diene-2,4,8-tricarboxylic acid 2,4-diethyl ester 8-methyl ester

| Conditions | Yield |
|---|---|
| rhodium(II) acetate | 62% |
-
-
872885-13-7
4-chloro-1,1,1-trifluorobut-3-yn-2-one

-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 20℃; for 6h; Diels-Alder Cycloaddition; Inert atmosphere; | A 19% B 62% |

-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
126554-33-4
ethyl (E)-2-diazo-4-(phenylsulfonyl)-3-butenoate

-
-
126554-29-8, 126554-42-5
(1S,4R,5R)-4-Benzenesulfonyl-8-aza-bicyclo[3.2.1]octa-2,6-diene-2,8-dicarboxylic acid 2-ethyl ester 8-methyl ester

| Conditions | Yield |
|---|---|
| rhodium(II) acetate | 61% |
-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
115419-61-9
N-[2,2,2-trichloro-1-(trimethylsilyloxy)ethyl]-N-(trimethylsilyl)amine

| Conditions | Yield |
|---|---|
| Stage #1: methyl 1H-pyrrole-1-carboxylate; N-[2,2,2-trichloro-1-(trimethylsilyloxy)ethyl]-N-(trimethylsilyl)amine With chloro-trimethyl-silane In dichloromethane at 20℃; for 0.05h; Stage #2: With hafnium tetrakis(trifluoromethanesulfonate) In dichloromethane at 20℃; for 10h; Further stages.; | 61% |
-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
54210-98-9
1,3-dibromo-1-phenyl-2-propanone

| Conditions | Yield |
|---|---|
| With diethylzinc In hexane; toluene | A 15% B 59% |


| Conditions | Yield |
|---|---|
| With sodium 3-{(4-methoxyphenyl)thio}propane-1-sulfonate; copper diacetate; palladium diacetate; toluene-4-sulfonic acid In N,N-dimethyl-formamide at 70℃; for 18h; | 59% |
-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
23680-40-2
methyl 3-bromopropiolate

-
-
208177-19-9
3-Bromo-7-aza-bicyclo[2.2.1]hepta-2,5-diene-2,7-dicarboxylic acid dimethyl ester

| Conditions | Yield |
|---|---|
| at 90 - 95℃; for 33h; | 56% |
-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
23680-40-2
methyl 3-bromopropiolate

| Conditions | Yield |
|---|---|
| at 90 - 95℃; for 30h; Cycloaddition; | 56% |
-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
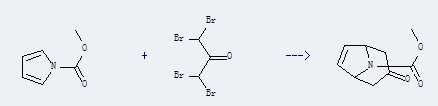
815-60-1
2,4-dibromo-3-pentanone

-
-
53406-52-3
methyl 2α,4α-dimethyl-3-oxo-8-azabicyclo<3.2.1>oct-6-ene-8-carboxylate

| Conditions | Yield |
|---|---|
| With diethylzinc In hexane; benzene a) 0 deg C, 3 h, b) r.t., 17 h; | 55% |
-
-
4277-63-8
methyl 1H-pyrrole-1-carboxylate

-
-
55591-23-6
1,1,2,2,3,3,4,4,5,5,6,6,6-tridecafluorohexane-1-sulfonyl chloride

| Conditions | Yield |
|---|---|
| With dichlorotris(triphenylphosphine)ruthenium(II) In pentane at 120℃; for 24h; | A 55% B 1% |

Methyl 1H-Pyrrole-1-carboxylate Specification
The cas register number of Methyl 1H-Pyrrole-1-carboxylate is 4277-63-8. It also can be called as 1H-Pyrrole-1-carboxylicacid, methyl ester and the IUPAC Name about this chemical is methyl pyrrole-1-carboxylate. It belongs to the following product categories, such as Carboxylic Acids, Pyrroles & Indoles, Carboxylic Acids, Pyrroles & Indoles, Building Blocks, Heterocyclic Building Blocks, Pyrroles and so on.
Physical properties about Methyl 1H-Pyrrole-1-carboxylate are: (1)ACD/LogP: 1.19; (2)ACD/LogD (pH 5.5): 1.19; (3)ACD/LogD (pH 7.4): 1.19; (4)ACD/BCF (pH 5.5): 4.75; (5)ACD/BCF (pH 7.4): 4.75; (6)ACD/KOC (pH 5.5): 106.16; (7)ACD/KOC (pH 7.4): 106.16; (8)#H bond acceptors: 3; (9)#Freely Rotating Bonds: 1; (10)Polar Surface Area: 31.23Å2; (11)Index of Refraction: 1.501; (12)Molar Refractivity: 33.65 cm3; (13)Molar Volume: 114 cm3; (14)Polarizability: 13.34x10-24cm3; (15)Surface Tension: 37.7 dyne/cm; (16)Enthalpy of Vaporization: 41.87 kJ/mol; (17)Vapour Pressure: 0.809 mmHg at 25°C.
Preparation: this chemical can be prepared by 1-methoxycarbonyl-2,5-dimethoxypyrrolidine. This reaction will need reagent TsOH and solvent benzene. The reaction time is 4 hour(s) at Heating. The yield is about 62%.

Uses of 1H-Pyrrole-1-carboxylate: it can be used to produce 3-oxo-nortrop-6-ene-8-carboxylic acid methyl ester with 1,1,3,3-tetrabromo-propan-2-one at temperature of -12 - 20 ℃. This reaction will need reagent ZnEt2 and solvent toluene with reaction time of 18 hours. The yield is about 40%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. When you are using it, please avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OC)n1cccc1
(2)InChI: InChI=1/C6H7NO2/c1-9-6(8)7-4-2-3-5-7/h2-5H,1H3
(3)InChIKey: MORALDOSFHZOQS-UHFFFAOYAL
(4)Std. InChI: InChI=1S/C6H7NO2/c1-9-6(8)7-4-2-3-5-7/h2-5H,1H3
(5)Std. InChIKey: MORALDOSFHZOQS-UHFFFAOYSA-N
Related Products
- Methyl 1-Benzyl-5-oxopyrrolidine-3-carboxylate
- Methyl (((methoxymethylphosphinothioyl)thio)acetyl)methylcarbamate
- Methyl (+)-(3R)-7-[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonylamino)pyrimidin-5-yl]-3-hydroxy-5-oxo-(6E)-heptenoate
- Methyl (2-amino-5-methyl-1,3-thiazol-4-yl)acetate
- Methyl (2-chloromethyl)oxazole-4-carboxylate
- Methyl (2E)-3-(4-methylphenyl)propenoate
- Methyl (2E)-3-cyclohexylprop-2-enoate
- Methyl (2R)-2-[(tert-butoxycarbonyl)amino]-3-iodopropanoate
- Methyl (2R)-2-[4-(2,4-dichlorophenoxy)phenoxy]propanoate
- Methyl (2R)-2-amino-2-cyclohexylethanoate hydrochloride
- 42779-56-6
- 42780-48-3
- 42783-04-0
- 42783-64-2
- 42783-78-8
- 42786-06-1
- 42787-75-7
- 427877-76-7
- 427878-02-2
- 42791-51-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View