-
Name
N-Acetyl-L-phenylalanine
- EINECS 217-959-8
- CAS No. 2018-61-3
- Article Data281
- CAS DataBase
- Density 1.199 g/cm3
- Solubility
- Melting Point 171-173 °C(lit.)
- Formula C11H13NO3
- Boiling Point 453.9 °C at 760 mmHg
- Molecular Weight 207.229
- Flash Point 228.3 °C
- Transport Information
- Appearance white to off-white fine cryst. powder or needles
- Safety 24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Acetylphenylalanine;(2S)-2-acetamido-3-phenyl-propanoic acid;Alanine, N-acetyl-3-phenyl-, L-;L-N-Acetylphenylalanine;Ac-Phe-OH;L-Phenylalanine, N-acetyl-;(2S)-2-acetamido-3-phenyl-propanoate;N-Acetyl-3-phenyl-L-alanine;Acetyl-L-phenylalanine;N-Acetylphenylalanine;Alanine, N-acetyl-3-phenyl-, L- (8CI);H-Phe-OH;
- PSA 66.40000
- LogP 1.20930
Synthetic route
-
-
55065-02-6, 64590-80-3, 5469-45-4
N-acetamido cinnamic acid

-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

| Conditions | Yield |
|---|---|
| With hydrogen; rhodium(I)-bis(1,5-cyclooctadiene) tetrafluoroborate; triethylamine; (2R,2'R)-bis(diphenylphosphino)-(1R,1'R)-dicyclopentane In tetrahydrofuran at 20℃; under 750.075 Torr; for 24h; | 100% |
| With hydrogen; (1+)*BF4(1-) In ethanol; benzene under 20 Torr; | 99% |
| With hydrogen; Rh(nbd)(1)BF4 In dichloromethane at 50℃; under 38787.1 Torr; for 12h; | 99% |
-
-
52386-78-4
N-acetyl dehydrophenylalanine methyl ester

-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

| Conditions | Yield |
|---|---|
| With hydrogen; bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate In methanol at 22℃; under 38000 Torr; | 100% |

| Conditions | Yield |
|---|---|
| With ammonium bicarbonate; water In dichloromethane for 12h; α-chymotrypsin; | 100% |
| With caesium carbonate In methanol; water for 24h; | 99% |
| With caesium carbonate In methanol; water for 24h; Product distribution; other alcohols; | 99% |
| With α-chymotrypsin In water at 25℃; Rate constant; pH 7.0-7.8; | |
| With bis-Tris propane buffer; calcium chloride at 25℃; Rate constant; |
-
-
63-91-2
L-phenylalanine

-
-
108-24-7
acetic anhydride

-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

| Conditions | Yield |
|---|---|
| In water for 0.0666667h; Irradiation; | 98% |
| With sodium hydroxide In water pH=10; Cooling with ice; | 93% |
| With sodium hydroxide In water at 20℃; for 3h; pH=8; Concentration; | 93% |

| Conditions | Yield |
|---|---|
| With magnesium iodide In tetrahydrofuran at 120℃; for 1h; Inert atmosphere; Microwave irradiation; Sealed tube; chemoselective reaction; | 98% |
| With sodium carbonate In methanol; water for 4h; | 88% |
| With sodium carbonate In methanol; water for 4h; Product distribution; other alkali carbonates; | 88% |
| With water In N,N-dimethyl-formamide carbonate buffer (pH=10.5); α-chymotrypsin-cat. hydrolysis (spec. activity); |

| Conditions | Yield |
|---|---|
| In water at 110℃; for 0.0833333h; Microwave irradiation; Green chemistry; chemoselective reaction; | 97% |
| With pyridine at 20℃; | 84.6% |

-
-
55065-02-6, 64590-80-3, 5469-45-4
N-acetamido cinnamic acid

-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

| Conditions | Yield |
|---|---|
| With hydrogen In tetrahydrofuran | 97% |

| Conditions | Yield |
|---|---|
| With hydrogen In methanol; benzene | 96.2% |
-
-
21156-62-7, 3618-96-0, 62436-70-8
N-acetylphenylalanine methyl ester

-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water Alcalase, pH 7.5; | 93.7% |
| With cross-linked yeast cells (Saccharomyces cerevisiae); water In dimethyl sulfoxide at 30℃; for 48h; |
-
-
21156-62-7, 3618-96-0, 62436-70-8
N-acetylphenylalanine methyl ester

-
A
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

-
B
-
21156-62-7
(R)-N-acetylphenylalanine methyl ester

| Conditions | Yield |
|---|---|
| With sodium hydroxide; phosphate buffer; pronase EC 3.4.24.4 In tetrahydrofuran Ambient temperature; | A 86% B 93% |
| Yield given. Yields of byproduct given; | |
| With Lecitase Ultra; water; calcium chloride at 30℃; for 12h; pH=8.5; aq. buffer; Enzymatic reaction; enantioselective reaction; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic anhydride In sodium hydrogencarbonate; ethyl acetate | 90.3% |
| With hydrogenchloride; acetic anhydride; triethylamine In water | |
| Multi-step reaction with 2 steps 1: dichloromethane / 16 h / 20 °C 2: dichloromethane / 16.5 h / -40 - 20 °C View Scheme |
-
-
55065-02-6, 64590-80-3, 5469-45-4
2-(N-acetylamino)cinnamic acid

-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

| Conditions | Yield |
|---|---|
| With bis(norbornadiene)rhodium(l)tetrafluoroborate; (3S,3'S)-3,3'-di-tert-butyl-2,2',3,3'-tetrahydro-4,4'-bibenzo[d][1,3]oxaphosphole; hydrogen In methanol at 20℃; under 5171.62 Torr; for 24h; Autoclave; enantioselective reaction; | 90% |
| With chloro(1,5-cyclooctadiene)rhodium(I) dimer; C48H42Fe2O2; hydrogen In methanol at 20℃; under 7600.51 Torr; for 24h; optical yield given as %ee; | 88% |
| With hydrogen In ethanol Product distribution; Ambient temperature; various catalysts; |
-
-
68277-06-5
(S)-N-acetylphenylalanine tert-butyl ester

-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid In dichloromethane at 20℃; for 6h; | 90% |

| Conditions | Yield |
|---|---|
| With copper(ll) sulfate pentahydrate In methanol at 20℃; for 0.0833333h; | 90% |
-
-
55065-02-6, 64590-80-3, 5469-45-4
N-acetamido cinnamic acid

-
A
-
10172-89-1
(R)-N-acetylphenylalanin

-
B
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

| Conditions | Yield |
|---|---|
| With [Rh(cod)(Xylophos)](1+)*BF4(1-); hydrogen In methanol at 19.85℃; under 760 Torr; for 6h; | A 11% B 89% |
| With hydrogen; (1+); Dowex HCR-S In methanol; water at 50℃; under 15001.2 Torr; Product distribution; other rhodium(I)-complexes, various reaction conditions: time, pressure and temperatures; other derivatives of acetamidoacryl acid; | |
| With hydrogen; chloro(1,5-cyclooctadiene)rhodium(I) dimer; optically active phosphine In methanol at 20 - 30℃; under 825.07 Torr; for 72h; Product distribution; various di- and triphosphines, other temperatures and times; |
-
-
103989-08-8, 104787-34-0
alpha-acetaminocinnamic acid

-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

| Conditions | Yield |
|---|---|
| 82% |

| Conditions | Yield |
|---|---|
| With (1-methyl-3-(3-sulfopropyl)-1H-imidazol-3-ium)3[PW12O403-] In neat (no solvent) at 120℃; for 0.333333h; Green chemistry; | 73% |
-
-
2885-12-3
L-lysine n-butyl ester

-
-
2361-96-8
ethyl N-acetyl-(L)-phenylalaninate

-
A
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

| Conditions | Yield |
|---|---|
| With Tris-HCl buffer; α-chymotropsin In water; N,N-dimethyl-formamide at 25℃; pH 9; | A n/a B 67% |

-
-
63-91-2
L-phenylalanine

-
-
108965-56-6
(4-acetoxyphenyl)-dimethylsulphonium methyl sulphate

-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

| Conditions | Yield |
|---|---|
| With triethylamine In water for 4h; Ambient temperature; pH=10.0; | 63% |
-
-
4117-33-3
L-Lysine ethyl ester

-
-
2361-96-8
ethyl N-acetyl-(L)-phenylalaninate

-
A
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

| Conditions | Yield |
|---|---|
| With Tris-HCl buffer; α-chymotropsin In water; N,N-dimethyl-formamide pH 9; | A n/a B 53% |
| With α-chymotrypsin at 25℃; for 0.05h; Product distribution; various buffer and pH; |

-
-
4134-09-2
ethyl N-acetyl-DL-phenylalaninate

-
A
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

-
B
-
2361-96-8, 4134-09-2, 57772-79-9, 20918-84-7
(R)-ethyl N-acetylphenylalanine

| Conditions | Yield |
|---|---|
| With ammonium bicarbonate; water In dichloromethane for 18h; α-chymotrypsin; | A 50% B 50% |
| A 50% B 50% | |
| In ethanol; water at 35℃; for 48h; Yield given; |
-
-
4134-09-2
ethyl N-acetyl-DL-phenylalaninate

-
A
-
10172-89-1
(R)-N-acetylphenylalanin

-
B
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

-
C
-
2361-96-8, 4134-09-2, 57772-79-9, 20918-84-7
(R)-ethyl N-acetylphenylalanine

| Conditions | Yield |
|---|---|
| With water In ethanol at 25℃; for 2h; Microbiological reaction; optical yield given as %ee; enantioselective reaction; | A n/a B n/a C 45% |
| at 35℃; for 48h; in the present of Saccharomyces cerevisiae Hansen; Yield given. Yields of byproduct given; |

-
-
42406-73-5
lysine benzyl ester p-toluenesulfonate salt

-
-
2361-96-8
ethyl N-acetyl-(L)-phenylalaninate

-
A
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

| Conditions | Yield |
|---|---|
| With Tris-HCl buffer; α-chymotropsin In water; N,N-dimethyl-formamide at 25℃; pH 9; | A n/a B 31% |

-
-
2901-75-9
(R,S)-N-acetyl phenylalanine

-
-
131348-01-1
(1S)-endo-fenchylamine

-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid
-
-
2901-75-9
(R,S)-N-acetyl phenylalanine

-
-
119-62-0, 716-61-0, 2792-51-0, 2792-52-1, 2964-48-9, 3689-55-2, 63526-31-8, 71115-69-0, 140694-64-0
(1S,2S)-2-amino-1-(4-nitrophenyl)propane-1,3-diol

-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

| Conditions | Yield |
|---|---|
| Reaktion ueber mehrere Stufen; |
-
-
5469-44-3
2-methyl-4-benzyl-4H-oxazolin-5-one

-
A
-
10172-89-1
(R)-N-acetylphenylalanin

-
B
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

| Conditions | Yield |
|---|---|
| With alpha cyclodextrin In water; acetonitrile pH 7.86; Title compound not separated from byproducts; |
-
-
55065-02-6, 64590-80-3, 5469-45-4
2-(N-acetylamino)cinnamic acid

-
A
-
10172-89-1
(R)-N-acetylphenylalanin

-
B
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

| Conditions | Yield |
|---|---|
| With hydrogen In methanol at 25℃; under 3150.3 Torr; for 4h; Product distribution; enantioselectivity of reaction dependent on various rhodium complexes with 1,2-bis(phosphanyl)pyrrolidine ligands; various conditions; also N-acetylcinnamic acid methylester; | |
| With 2S-MeN(PPh2)CHC7H7CH2OPPh2; hydrogen; Rh<(COD)Cl>2 Product distribution; other reagents; | |
| With hydrogen; bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; Br>n In ethanol at 20℃; Product distribution; asymmetric catalytic hydrogenation, enantiodifferentiating ability of the catalysts: atmospheric pressure of H2; var. catalysts, pressure, temp. and initial rate; |

| Conditions | Yield |
|---|---|
| In water α-chymotrypsin, 0.05 M phosphate buffer pH 7.4; |
-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

-
-
100400-96-2, 97290-54-5
(S)-2-acetylamino-3-cyclohexylpropionic acid

| Conditions | Yield |
|---|---|
| With platinum(IV) oxide; hydrogen; acetic acid In methanol under 2585.81 Torr; for 24h; | 100% |
| With hydrogen; rhodium on alumina In methanol for 56h; Ambient temperature; Pressure (range begins): 2.5 ; | 98.1% |
| With C33H49ClNRh; hydrogen In 2,2,2-trifluoroethanol at 20℃; under 45004.5 Torr; for 24h; Autoclave; Molecular sieve; | 93% |
| With acetic acid; platinum Hydrogenation; | |
| With sodium tetrahydroborate; rhodium(III) chloride 30 deg C, water, pH=4; Yield given. Multistep reaction; |
-
-
743477-27-2
2-hydroxy-1,1,2-trimethylpropyl ethylcarbamate

-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

-
A
-
19424-29-4
4,4,5,5-tetramethyl-1,3-dioxolan-2-one

-
B
-
2361-96-8
ethyl N-acetyl-(L)-phenylalaninate

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite; 3 A molecular sieve In dichloromethane at 60℃; for 12h; | A n/a B 100% |

-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

-
-
76290-84-1
ethyl-(2-hydroxy-ethyl)-dimethyl-ammonium; hydroxide

| Conditions | Yield |
|---|---|
| In water at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In water at 20℃; | 100% |
-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

-
-
114380-13-1
butyl(2-hydroxyethyl)dimethylammonium hydroxide

| Conditions | Yield |
|---|---|
| In water at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In water at 20℃; | 100% |
-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

-
-
75-03-6
ethyl iodide

-
-
2361-96-8
ethyl N-acetyl-(L)-phenylalaninate

| Conditions | Yield |
|---|---|
| With caesium carbonate In acetonitrile for 1.5h; Heating; | 99% |
| With cesium fluoride In N,N-dimethyl-formamide at 15℃; for 24h; | 90% |
-
-
6066-82-6
1-hydroxy-pyrrolidine-2,5-dione

-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

-
-
55604-95-0
N-acetyl-L-phenylalanine N-hydroxysuccinimide ester

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In dichloromethane at 0℃; for 3.5h; | 99% |
| With dicyclohexyl-carbodiimide In tetrahydrofuran at 0℃; | 85% |
| With dicyclohexyl-carbodiimide at 0 - 20℃; |
-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

-
-
15100-75-1
L-phenylalanine tert-butyl ester hydrochloride

-
-
128992-42-7
N-Acetylphenylalanylphenylalanine tert-butyl ester

| Conditions | Yield |
|---|---|
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 23h; | 99% |
-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

-
-
378784-00-0, 7440-16-6
rhodium

-
-
100400-96-2, 97290-54-5
(S)-2-acetylamino-3-cyclohexylpropionic acid

| Conditions | Yield |
|---|---|
| In methanol | 98.1% |

| Conditions | Yield |
|---|---|
| With pepsin immobilized on terephthalaldehyde functionalized chitosan magnetic nanoparticle In acetonitrile at 20℃; for 48h; pH=2; | 98% |
| With hydrogenchloride | |
| With hydrogen bromide |

| Conditions | Yield |
|---|---|
| With caesium carbonate In acetonitrile for 1.5h; Heating; | 98% |
-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

-
-
10606-72-1
(R)-mandelic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran for 6h; Mitsunobu reaction; | 98% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 20℃; for 4.5h; | 96% |
-
-
67-56-1
methanol

-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

-
-
3618-96-0
(S)-N-acetylphenylalanine

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride | 95% |
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione at 70℃; for 20h; | 95% |
| With N-Bromosuccinimide at 70℃; for 20h; | 91% |
-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

-
-
2562-48-3
N-acetyl-L-phenylalanyl-L-phenylalanine methylester

| Conditions | Yield |
|---|---|
| With 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride | 95% |
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane at 25℃; for 12h; | 53% |
| With sodium hydroxide; thermolysin at 37℃; for 24h; pH=6.3; enzymatic amidation; |
-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

-
-
849770-98-5
2-amino-3-[4-(tert-butoxycarbonylamino-methyl)-5-methoxymethoxy-6-methyl-pyridin-3-ylmethylsulfanyl]-propionic acid ethyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate | 95% |
-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

-
-
20667-12-3
silver(l) oxide

| Conditions | Yield |
|---|---|
| In acetonitrile slurry (S)-N-acetylphenylalanine in MeCN was added to slid Ag2O and stirred for 24 h in the dark; ppt. was filtered off, washed with Et2O, and dried in vacuo; elem. anal.; | 95% |
-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

-
-
147092-06-6
(L)-N-acetyl-phenylalanine vinyl ester

| Conditions | Yield |
|---|---|
| With N,N'-diethylurea; copper(II) bis(trifluoromethanesulfonate); triethylamine In tetrahydrofuran at 50℃; for 16h; Chan-Lam Coupling; | 94% |
-
-
2018-61-3
(S)-2-acetylamino-3-phenylpropanoic acid

-
-
107-14-2
chloroacetonitrile

-
-
61781-58-6
(S)-cyanomethyl 2-acetamido-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 0 - 20℃; for 4.5h; | 93% |
| With N-ethyl-N,N-diisopropylamine for 12h; | 90% |
| With N-ethyl-N,N-diisopropylamine for 12h; | 90% |
| With triethylamine In acetonitrile at 20℃; for 16h; Inert atmosphere; | 89% |
| With triethylamine |
N-Acetyl-L-phenylalanine Chemical Properties
Molecule structure of N-Acetyl-L-phenylalanine (CAS NO.2018-61-3):
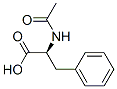
IUPAC Name: (2S)-2-Acetamido-3-phenylpropanoic acid
Molecular Weight: 207.22582 g/mol
Molecular Formula: C11H13NO3
Density: 1.199 g/cm3
Melting Point: 171-173 °C(lit.)
Boiling Point: 453.9 °C at 760 mmHg
Flash Point: 228.3 °C
Index of Refraction: 1.547
Molar Refractivity: 54.84 cm3
Molar Volume: 172.7 cm3
Surface Tension: 48.3 dyne/cm
Enthalpy of Vaporization: 75.18 kJ/mol
Vapour Pressure: 4.96E-09 mmHg at 25 °C
XLogP3: 0.6
H-Bond Donor: 2
H-Bond Acceptor: 3
Rotatable Bond Count: 4
Tautomer Count: 2
Exact Mass: 207.089543
MonoIsotopic Mass: 207.089543
Topological Polar Surface Area: 66.4
Heavy Atom Count: 15
Canonical SMILES: CC(=O)NC(CC1=CC=CC=C1)C(=O)O
Isomeric SMILES: CC(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O
InChI: InChI=1S/C11H13NO3/c1-8(13)12-10(11(14)15)7-9-5-3-2-4-6-9/h2-6,10H,7H2,1H3,(H,12,13)(H,14,15)/t10-/m0/s1
InChIKey: CBQJSKKFNMDLON-JTQLQIEISA-N
EINECS: 217-959-8
Product Categories: Amino Acids Derivatives; chiral; Amino Acid Derivatives; Amino Acids; Phenylalanine [Phe, F]; Amino Acids and Derivatives; Ac-Amino Acids; Amino Acids (N-Protected); Biochemistry; N-Acetyl-Amino acid series; Amino Acid Derivatives; Peptide Synthesis; Phenylalanine
N-Acetyl-L-phenylalanine Safety Profile
Safety Statements of N-Acetyl-L-phenylalanine (CAS NO.2018-61-3): 24/25
S24/25: Avoid contact with skin and eyes.
WGK Germany: 3
N-Acetyl-L-phenylalanine Specification
N-Acetyl-L-phenylalanine (CAS NO.2018-61-3) is also named as Acetyl-L-phenylalanine ; Acetylphenylalanine ; L-N-Acetylphenylalanine ; N-Acetylphenylalanine ; NSC 45699 ; Alanine, N-acetyl-3-phenyl-, L- (8CI) ; L-Phenylalanine, N-acetyl- ; N-Acetyl-3-phenyl-L-alanine . It is white to off-white fine cryst. powder or needles.
Related Products
- N-Acetyl-L-phenylalanine
- 201864-71-3
- 2018-66-8
- 20187-46-6
- 20187-55-7
- 201-88-7
- 2018-90-8
- 20189-11-1
- 20191-74-6
- 201928-42-9
- 201929-84-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View