-
Name
Z-ORN(BOC)-OH
- EINECS 1592732-453-0
- CAS No. 7733-29-1
- Article Data15
- CAS DataBase
- Density 1.193 g/cm3
- Solubility
- Melting Point 96-103ºC
- Formula C18H26N2O6
- Boiling Point 579.1 °C at 760 mmHg
- Molecular Weight 366.414
- Flash Point 304 °C
- Transport Information
- Appearance white - off-white powder
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Ornithine,N2,N5-dicarboxy-, N2-benzyl N5-tert-butyl ester, L- (7CI,8CI);(2S)-5-((tert-Butoxycarbonyl)amino)-2-((benzyloxycarbonyl)amino)pentanoicacid;N-a-Benzyloxycarbonyl-N-d-tert-butoxycarbonyl-L-ornithine;NSC 336232;
- PSA 113.96000
- LogP 3.45270
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: copper(II) complex of Nδ-tert-butoxycarbonyl-L-ornithine With 8-quinolinol; sodium carbonate In acetone for 1h; Stage #2: benzyl chloroformate With 1-hydroxy-pyrrolidine-2,5-dione In water at -5℃; for 0.5h; | 95% |
-
-
13650-49-2
Nδ-(tert-butoxycarbonyl)-L-ornithine

-
-
501-53-1
benzyl chloroformate

-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water at 20℃; for 12h; | 90% |
-
-
19506-72-0
benzyl-8-quinolyl carbonate

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
3184-13-2
L-ornithine hydrochloride

-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| Acylation; Multistep reaction; | 82% |

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
2640-58-6
Nα-benzyloxycarbonyl-L-ornithine

-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| With sodium hydroxide; triethylamine In methanol at 20℃; for 24h; | 36% |
| With triethylamine In methanol at 40℃; for 1h; | |
| With sodium hydrogencarbonate |
-
-
1070-19-5
N-(tert-butyloxycarbonyl) azide

-
-
2640-58-6
Nα-benzyloxycarbonyl-L-ornithine

-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| With hydroxide | |
| With magnesium oxide In 1,4-dioxane; water |
-
-
16965-08-5
1,1-dimethylethyl 2,4,5-trichlorophenyl carbonate

-
-
62234-40-6
(2S)-2-{[(benzyloxy)carbonyl]amino}-4-(dimethylamino)butanoic acid

-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| With triethylamine In tert-butyl alcohol |

| Conditions | Yield |
|---|---|
| With sodium hydroxide |
-
-
13139-17-8
N-(Benzyloxycarbonyloxy)succinimide

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
3184-13-2
L-ornithine hydrochloride

-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| Stage #1: di-tert-butyl dicarbonate; L-ornithine hydrochloride With sodium hydroxide; copper diacetate In water; acetone for 44h; Stage #2: With 8-quinolinol; sodium carbonate In water; acetone for 1.5h; Stage #3: N-(Benzyloxycarbonyloxy)succinimide In water; acetone at 20℃; for 1.5h; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: Na2CO3 / H2O; acetone / 0.5 h / -10 °C 2.1: Cu(CH3COO)2; NaOH / H2O; acetone / 44 h 2.2: 8-quinolinol; Na2CO3 / acetone; H2O / 1.5 h 2.3: H2O; acetone / 1.5 h / 20 °C View Scheme |
-
-
25693-00-9
N5-((Ξ)-benzyliden)-L-ornithine

-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: MgO / dioxane; H2O View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: MgO / dioxane; H2O View Scheme |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: copper(II) View Scheme |

| Conditions | Yield |
|---|---|
| With triethylsilane; trifluoroacetic acid In dichloromethane for 0.166667h; Ambient temperature; | 100% |
| With trifluoroacetic acid In dichloromethane for 0.5h; | 92% |
| With toluene-4-sulfonic acid; triethylamine In acetone for 1h; Heating; | 19.55 g |

| Conditions | Yield |
|---|---|
| Stage #1: Z-Orn(Boc)-OH With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 0.166667h; Stage #2: L-valine methylester hydrochloride In N,N-dimethyl-formamide at 20℃; for 18h; Enzymatic reaction; | 99% |
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; triethylamine In N,N-dimethyl-formamide at 20℃; | 86% |
-
-
7733-29-1
Z-Orn(Boc)-OH

-
-
119158-00-8
(S)-benzyl 1-amino-5-tert-butoxycarbonylamino-1-oxopentan-2-yl-ylcarbamate

| Conditions | Yield |
|---|---|
| Stage #1: Z-Orn(Boc)-OH With 4-methyl-morpholine; isobutyl chloroformate In tetrahydrofuran at -10℃; for 0.333333h; Inert atmosphere; Stage #2: With ammonium hydroxide In tetrahydrofuran; water at 10 - 20℃; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: Z-Orn(Boc)-OH With benzotriazol-1-ol; dicyclohexyl-carbodiimide In tetrahydrofuran for 0.166667h; Cooling with ice; Stage #2: benzylamine With 4-methyl-morpholine at 20℃; for 12h; pH=8; Cooling with ice; | 98.7% |
| Stage #1: Z-Orn(Boc)-OH With benzotriazol-1-ol; dicyclohexyl-carbodiimide In tetrahydrofuran for 0.166667h; Cooling with ice; Stage #2: benzylamine With 4-methyl-morpholine at 20℃; for 8h; pH=8; | 98.7% |
-
-
7733-29-1
Z-Orn(Boc)-OH

-
-
6638-79-5
N,O-dimethylhydroxylamine*hydrochloride

-
-
913094-50-5
[4-benzyloxycarbonylamino-4-(methoxy-methyl-carbamoyl)-butyl]-carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: Z-Orn(Boc)-OH; N,O-dimethylhydroxylamine*hydrochloride With N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 0℃; for 0.0833333h; Stage #2: With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 2h; | 98% |
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; triethylamine In N,N-dimethyl-formamide at 0℃; for 3h; | 95% |

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; triethylamine In N,N-dimethyl-formamide at 20℃; | 98% |
| With 2,6-dimethylpyridine; 1-[(1-(cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino)]-uronium hexafluorophosphate In water at 20 - 25℃; | 91% |
| Stage #1: Z-Orn(Boc)-OH With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 0.25h; Inert atmosphere; Stage #2: methyl (L)-leucinate hydrochloride In N,N-dimethyl-formamide at 20℃; for 12h; Inert atmosphere; | 86% |

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; triethylamine In N,N-dimethyl-formamide at 20℃; | 98% |
-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| Stage #1: Boc-threo-β-phenyl-Ser-Sta-NH(CH2)2Ph With hydrogenchloride In 1,4-dioxane at 20℃; for 1h; Stage #2: Z-Orn(Boc)-OH With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide for 18h; | 98% |

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; triethylamine In N,N-dimethyl-formamide at 20℃; | 97% |

| Conditions | Yield |
|---|---|
| 96% |
-
-
7733-29-1
Z-Orn(Boc)-OH

-
-
169157-66-8
(2,2-dimethoxyethyl)aminoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In dichloromethane at 20℃; for 2h; | 94% |
-
-
57260-73-8
N-BOC-1,2-diaminoethane

-
-
7733-29-1
Z-Orn(Boc)-OH

-
-
33194-29-5
benzyl-{(1S)-4-[(tert-butoxycarbonyl)amino]-1-[({2-[(tert-butoxycarbonyl)amino]ethyl}amino)-carbonyl]butyl}carbamate

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In water; N,N-dimethyl-formamide at 0 - 20℃; for 12h; | 94% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 20℃; for 12h; | 94% |
-
-
7733-29-1
Z-Orn(Boc)-OH

-
-
92762-94-2
β-benzyl-L-aspartic acid amide trifluoroacetate

-
-
129594-14-5
Z-Orn(Boc)-Asp(OBzl)-NH2

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; triethylamine; dicyclohexyl-carbodiimide In tetrahydrofuran 1.) 0 deg C, 30 min, 2.) RT, 4 h; | 90% |
-
-
7733-29-1
Z-Orn(Boc)-OH

-
-
100-39-0
benzyl bromide

-
-
92455-57-7
Nα-(Benzyloxycarbonyl)-Nδ-(t-butoxycarbonyl)-L-ornithine benzyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In ethyl acetate for 8h; Heating; | 90% |
| With triethylamine In N,N-dimethyl-formamide at 20℃; for 24h; | 79% |
| With diisopropylamine In acetonitrile at 20℃; for 14h; | 7.89 g |
-
-
7733-29-1
Z-Orn(Boc)-OH

-
-
331866-80-9
(R)-3-Amino-hexadecanoic acid methyl ester

-
-
331866-81-0
(R)-3-((S)-2-Benzyloxycarbonylamino-5-tert-butoxycarbonylamino-pentanoylamino)-hexadecanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 2h; | 90% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In chloroform at 0℃; for 2h; | 89% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| Stage #1: Z-Orn(Boc)-OH With triethylamine; isobutyl chloroformate In tetrahydrofuran Stage #2: diazomethane In tetrahydrofuran; diethyl ether at 4℃; Stage #3: With hydrogenchloride In tetrahydrofuran; 1,4-dioxane; diethyl ether at -15℃; for 0.25h; | 88.9% |

| Conditions | Yield |
|---|---|
| Stage #1: copper(II) complex of Nδ-tert-butoxycarbonyl-L-ornithine With 8-quinolinol; sodium carbonate In acetone for 1h; Stage #2: benzyl chloroformate With 1-hydroxy-pyrrolidine-2,5-dione In water at -5℃; for 0.5h; | 95% |
-
-
13650-49-2
Nδ-(tert-butoxycarbonyl)-L-ornithine

-
-
501-53-1
benzyl chloroformate

-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water at 20℃; for 12h; | 90% |
-
-
19506-72-0
benzyl-8-quinolyl carbonate

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
3184-13-2
L-ornithine hydrochloride

-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| Acylation; Multistep reaction; | 82% |

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
2640-58-6
Nα-benzyloxycarbonyl-L-ornithine

-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| With sodium hydroxide; triethylamine In methanol at 20℃; for 24h; | 36% |
| With triethylamine In methanol at 40℃; for 1h; | |
| With sodium hydrogencarbonate |
-
-
1070-19-5
N-(tert-butyloxycarbonyl) azide

-
-
2640-58-6
Nα-benzyloxycarbonyl-L-ornithine

-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| With hydroxide | |
| With magnesium oxide In 1,4-dioxane; water |
-
-
16965-08-5
1,1-dimethylethyl 2,4,5-trichlorophenyl carbonate

-
-
62234-40-6
(2S)-2-{[(benzyloxy)carbonyl]amino}-4-(dimethylamino)butanoic acid

-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| With triethylamine In tert-butyl alcohol |

| Conditions | Yield |
|---|---|
| With sodium hydroxide |
-
-
13139-17-8
N-(Benzyloxycarbonyloxy)succinimide

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
3184-13-2
L-ornithine hydrochloride

-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| Stage #1: di-tert-butyl dicarbonate; L-ornithine hydrochloride With sodium hydroxide; copper diacetate In water; acetone for 44h; Stage #2: With 8-quinolinol; sodium carbonate In water; acetone for 1.5h; Stage #3: N-(Benzyloxycarbonyloxy)succinimide In water; acetone at 20℃; for 1.5h; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: Na2CO3 / H2O; acetone / 0.5 h / -10 °C 2.1: Cu(CH3COO)2; NaOH / H2O; acetone / 44 h 2.2: 8-quinolinol; Na2CO3 / acetone; H2O / 1.5 h 2.3: H2O; acetone / 1.5 h / 20 °C View Scheme |
-
-
25693-00-9
N5-((Ξ)-benzyliden)-L-ornithine

-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: MgO / dioxane; H2O View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: MgO / dioxane; H2O View Scheme |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: copper(II) View Scheme |

| Conditions | Yield |
|---|---|
| With triethylsilane; trifluoroacetic acid In dichloromethane for 0.166667h; Ambient temperature; | 100% |
| With trifluoroacetic acid In dichloromethane for 0.5h; | 92% |
| With toluene-4-sulfonic acid; triethylamine In acetone for 1h; Heating; | 19.55 g |

| Conditions | Yield |
|---|---|
| Stage #1: Z-Orn(Boc)-OH With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 0.166667h; Stage #2: L-valine methylester hydrochloride In N,N-dimethyl-formamide at 20℃; for 18h; Enzymatic reaction; | 99% |
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; triethylamine In N,N-dimethyl-formamide at 20℃; | 86% |
-
-
7733-29-1
Z-Orn(Boc)-OH

-
-
119158-00-8
(S)-benzyl 1-amino-5-tert-butoxycarbonylamino-1-oxopentan-2-yl-ylcarbamate

| Conditions | Yield |
|---|---|
| Stage #1: Z-Orn(Boc)-OH With 4-methyl-morpholine; isobutyl chloroformate In tetrahydrofuran at -10℃; for 0.333333h; Inert atmosphere; Stage #2: With ammonium hydroxide In tetrahydrofuran; water at 10 - 20℃; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: Z-Orn(Boc)-OH With benzotriazol-1-ol; dicyclohexyl-carbodiimide In tetrahydrofuran for 0.166667h; Cooling with ice; Stage #2: benzylamine With 4-methyl-morpholine at 20℃; for 12h; pH=8; Cooling with ice; | 98.7% |
| Stage #1: Z-Orn(Boc)-OH With benzotriazol-1-ol; dicyclohexyl-carbodiimide In tetrahydrofuran for 0.166667h; Cooling with ice; Stage #2: benzylamine With 4-methyl-morpholine at 20℃; for 8h; pH=8; | 98.7% |
-
-
7733-29-1
Z-Orn(Boc)-OH

-
-
6638-79-5
N,O-dimethylhydroxylamine*hydrochloride

-
-
913094-50-5
[4-benzyloxycarbonylamino-4-(methoxy-methyl-carbamoyl)-butyl]-carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: Z-Orn(Boc)-OH; N,O-dimethylhydroxylamine*hydrochloride With N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 0℃; for 0.0833333h; Stage #2: With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 2h; | 98% |
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; triethylamine In N,N-dimethyl-formamide at 0℃; for 3h; | 95% |

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; triethylamine In N,N-dimethyl-formamide at 20℃; | 98% |
| With 2,6-dimethylpyridine; 1-[(1-(cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino)]-uronium hexafluorophosphate In water at 20 - 25℃; | 91% |
| Stage #1: Z-Orn(Boc)-OH With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 0.25h; Inert atmosphere; Stage #2: methyl (L)-leucinate hydrochloride In N,N-dimethyl-formamide at 20℃; for 12h; Inert atmosphere; | 86% |

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; triethylamine In N,N-dimethyl-formamide at 20℃; | 98% |
-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| Stage #1: Boc-threo-β-phenyl-Ser-Sta-NH(CH2)2Ph With hydrogenchloride In 1,4-dioxane at 20℃; for 1h; Stage #2: Z-Orn(Boc)-OH With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide for 18h; | 98% |

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; triethylamine In N,N-dimethyl-formamide at 20℃; | 97% |

| Conditions | Yield |
|---|---|
| 96% |
-
-
7733-29-1
Z-Orn(Boc)-OH

-
-
169157-66-8
(2,2-dimethoxyethyl)aminoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In dichloromethane at 20℃; for 2h; | 94% |
-
-
57260-73-8
N-BOC-1,2-diaminoethane

-
-
7733-29-1
Z-Orn(Boc)-OH

-
-
33194-29-5
benzyl-{(1S)-4-[(tert-butoxycarbonyl)amino]-1-[({2-[(tert-butoxycarbonyl)amino]ethyl}amino)-carbonyl]butyl}carbamate

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In water; N,N-dimethyl-formamide at 0 - 20℃; for 12h; | 94% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 20℃; for 12h; | 94% |
-
-
7733-29-1
Z-Orn(Boc)-OH

-
-
92762-94-2
β-benzyl-L-aspartic acid amide trifluoroacetate

-
-
129594-14-5
Z-Orn(Boc)-Asp(OBzl)-NH2

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; triethylamine; dicyclohexyl-carbodiimide In tetrahydrofuran 1.) 0 deg C, 30 min, 2.) RT, 4 h; | 90% |
-
-
7733-29-1
Z-Orn(Boc)-OH

-
-
100-39-0
benzyl bromide

-
-
92455-57-7
Nα-(Benzyloxycarbonyl)-Nδ-(t-butoxycarbonyl)-L-ornithine benzyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In ethyl acetate for 8h; Heating; | 90% |
| With triethylamine In N,N-dimethyl-formamide at 20℃; for 24h; | 79% |
| With diisopropylamine In acetonitrile at 20℃; for 14h; | 7.89 g |
-
-
7733-29-1
Z-Orn(Boc)-OH

-
-
331866-80-9
(R)-3-Amino-hexadecanoic acid methyl ester

-
-
331866-81-0
(R)-3-((S)-2-Benzyloxycarbonylamino-5-tert-butoxycarbonylamino-pentanoylamino)-hexadecanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 2h; | 90% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In chloroform at 0℃; for 2h; | 89% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
7733-29-1
Z-Orn(Boc)-OH

| Conditions | Yield |
|---|---|
| Stage #1: Z-Orn(Boc)-OH With triethylamine; isobutyl chloroformate In tetrahydrofuran Stage #2: diazomethane In tetrahydrofuran; diethyl ether at 4℃; Stage #3: With hydrogenchloride In tetrahydrofuran; 1,4-dioxane; diethyl ether at -15℃; for 0.25h; | 88.9% |
N-Cbz-N'-Boc-L-ornithine Chemical Properties
Molar mass:366.40884g/mol
Structure of Z-ORN(BOC)-OH(7733-29-1):
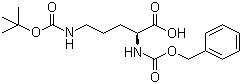
Synonyms of Z-ORN(BOC)-OH(7733-29-1):CBZ-ORN(BOC)-OH;N-ALPHA-BENZYLOXYCARBONYL-N-DELTA-TERT-BUTYLOXYCARBONYL-L-ORNITHINE;N-ALPHA-BENZYLOXYCARBONYL-N-DELTA-T-BUTYLOXYCARBONYL-L-ORNITHINE;N-ALPHA-BENZYLOXYCARBONYL-N-DELTA-BOC-L-ORNITHINE;N-ALPHA-CARBOBENZOXY,N-DELTA-T-BUTOXYCARBONYL-L-ORNITHINE;Z-N-S-BOC-L-ORNITHINE;N-ALPHA-CBZ-N-DELTA-T-BOC-L-ORNITHINE;Z-N-DELTA-BOC-L-ORNITHINE
Density:1.193 g/cm3
Flash Point:304 °C
Boiling Point:579.1 °C at 760 mmHg
Index of Refraction:1.526
Vapour Pressure:2.99E-14 mmHg at 25°C
N-Cbz-N'-Boc-L-ornithine Specification
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View