-
Name
N-Phenyl-bis(trifluoromethanesulfonimide)
- EINECS 609-445-0
- CAS No. 37595-74-7
- Article Data23
- CAS DataBase
- Density 1.766 g/cm3
- Solubility solubile in methanol
- Melting Point 100-102 °C
- Formula C8H5F6NO4S2
- Boiling Point 305.3 °C at 760 mmHg
- Molecular Weight 357.254
- Flash Point 138.4 °C
- Transport Information
- Appearance white to off-white crystalline powder
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1,1,1-Trifluoro-N-phenyl-N-[(trifluoromethyl)sulfonyl]methanesulfonamide;N-Phenyl-N-[(trifluoromethyl)sulfonyl]trifluoromethanesulfonamide;N-Phenylbis(trifluoromethanesulfonimide);N-Phenyltriflimide;N-Phenyltrifluoromethylsulfonimide;Phenyl triflimide;Trifluoro-N-phenyl-N-[(trifluoromethyl)sulfonyl]methanesulfonamide;N-Phenylbis(trifluoromethanesulphonimide);
- PSA 88.28000
- LogP 4.35380
Synthetic route
-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
62-53-3
aniline

-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

| Conditions | Yield |
|---|---|
| Stage #1: trifluoromethylsulfonic anhydride; aniline With 1,4-diaza-bicyclo[2.2.2]octane In acetonitrile at 40℃; under 600.06 - 1725.17 Torr; for 8h; Sealed tube; Large scale; Stage #2: With dmap In dichloromethane; acetonitrile under 450.045 - 1575.16 Torr; for 15h; Sealed tube; Large scale; | 94% |
| With triethylamine In dichloromethane at 25℃; for 16h; Inert atmosphere; Industry scale; Cooling with ice; | 86% |
| With triethylamine In dichloromethane at 20℃; Cooling with ice; | 86% |

-
-
82113-65-3
bis(trifluoromethanesulfonyl)amide

-
-
71-43-2
benzene

-
A
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

| Conditions | Yield |
|---|---|
| at 120℃; Glovebox; | A 7%Spectr. B 93% |

-
-
335-05-7
Trifluoromethanesulfonyl fluoride

-
-
62-53-3
aniline

-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

| Conditions | Yield |
|---|---|
| Stage #1: Trifluoromethanesulfonyl fluoride; aniline With 1,8-diazabicyclo[5.4.0]undec-7-ene In N,N-dimethyl-formamide at 60℃; under 675.068 Torr; for 8h; Sealed tube; Large scale; Stage #2: With dmap In toluene at 60℃; under 750.075 - 1950.2 Torr; for 10h; Pressure; Solvent; Sealed tube; Large scale; | 92.1% |
| With dmap; potassium carbonate In dichloromethane at -40 - 0℃; under 150.015 - 750.075 Torr; for 12h; Reagent/catalyst; | 90.7% |

-
-
421-83-0
trifluoromethane sulfonyl chloride

-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran | 60% |
| In tetrahydrofuran | 60% |

-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

| Conditions | Yield |
|---|---|
| In freon 113=1,1,2-trichloro-trifluoroethane at 95°C; | 34% |
| In 1,1,2-Trichloro-1,2,2-trifluoroethane |
-
-
369-57-3
benzenediazonium tetrafluoroborate

-
-
174899-83-3
1-butyl-3-methylimidazolium trifluoromethanesulfonimide

-
B
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

| Conditions | Yield |
|---|---|
| With [Bmim][Br] at 24.84℃; for 24h; Kinetics; | |
| at 24.84℃; for 24h; Kinetics; |

-
-
369-57-3
benzenediazonium tetrafluoroborate

-
-
174899-83-3
1-butyl-3-methylimidazolium trifluoromethanesulfonimide

-
B
-
462-06-6
fluorobenzene

-
C
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

| Conditions | Yield |
|---|---|
| at 90℃; for 0.216667h; |

-
-
1025373-45-8
trifluoromethanesulfonic acid anhydride

-
-
62-53-3
aniline

-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at -78 - 20℃; Inert atmosphere; |
-
-
421-83-0
trifluoromethane sulfonyl chloride

-
-
62-53-3
aniline

-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 2h; |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
38923-57-8
methyl 2,2-dimethyl-3-oxobutanoate

-
-
143225-17-6
methyl 2,2-dimethyl-3-0-(trifluoromethanesulfonate)-but-3-enoate

| Conditions | Yield |
|---|---|
| Stage #1: methyl 2,2-dimethyl-3-oxobutanoate With lithium diisopropyl amide In tetrahydrofuran; hexane at -78℃; for 0.25h; Inert atmosphere; Stage #2: N,N-phenylbistrifluoromethane-sulfonimide In tetrahydrofuran; hexane at -78℃; for 1.25h; Inert atmosphere; | 100% |
| Stage #1: methyl 2,2-dimethyl-3-oxobutanoate With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -20℃; for 0.166667h; Stage #2: N,N-phenylbistrifluoromethane-sulfonimide at -10℃; for 2h; | 87% |
| With lithium diisopropyl amide In tetrahydrofuran -78 deg C to RT; | |
| Stage #1: methyl 2,2-dimethyl-3-oxobutanoate With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -78℃; for 0.258333h; Inert atmosphere; Stage #2: N,N-phenylbistrifluoromethane-sulfonimide In tetrahydrofuran; hexane Inert atmosphere; |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
120566-50-9
(1RS,2RS,6SR,7RS,8SR)-7-bromo-3-oxo-1-vinyl-9-azatricyclo[4.4.0.02,8]decane-9-carboxylic acid methyl ester

-
-
120566-52-1
(1RS,2RS,6SR,7RS,8SR)-7-bromo-3-trifluoromethanesulfonyloxy-1-vinyl-9-azatricyclo[4.4.0.02,8]dec-3-ene-9-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at -78 - 0℃; | 100% |
| Stage #1: (1RS,2RS,6SR,7RS,8SR)-7-bromo-3-oxo-1-vinyl-9-azatricyclo[4.4.0.02,8]decane-9-carboxylic acid methyl ester With lithium diisopropyl amide In tetrahydrofuran; hexane at -78℃; for 1h; Stage #2: N,N-phenylbistrifluoromethane-sulfonimide In tetrahydrofuran; hexane at -78 - 0℃; |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
1078-19-9
6-methoxy-3,4-dihydro-1(2H)-naphthalenone

-
-
115375-59-2
6-methoxy-3,4-dihydronaphthalene-1-yl triflate

| Conditions | Yield |
|---|---|
| Stage #1: 6-methoxy-3,4-dihydro-1(2H)-naphthalenone With lithium hexamethyldisilazane In tetrahydrofuran at -78℃; for 0.75h; Stage #2: N,N-phenylbistrifluoromethane-sulfonimide In tetrahydrofuran at -78 - 0℃; for 3h; | 100% |
| With lithium hexamethyldisilazane In tetrahydrofuran at -78 - 20℃; for 16h; Inert atmosphere; | 89% |
| Stage #1: 6-methoxy-3,4-dihydro-1(2H)-naphthalenone With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 1h; Inert atmosphere; Stage #2: N,N-phenylbistrifluoromethane-sulfonimide In tetrahydrofuran at -78 - 20℃; Inert atmosphere; | 80% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
42058-59-3
3-hydroxy-benzeneacetic acid, methyl ester

-
-
147283-87-2
methyl [3-[[(trifluoromethyl)sulfonyl]oxy]phenyl]-acetate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane Ambient temperature; | 100% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
1193-47-1
2,2-dimethyl-cyclohexanone

-
-
32363-23-8
6,6-dimethylcyclohex-1-en-1-yl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| Stage #1: 2,2-dimethyl-cyclohexanone With lithium diisopropyl amide In tetrahydrofuran at -78℃; Stage #2: N,N-phenylbistrifluoromethane-sulfonimide In tetrahydrofuran at -78℃; | 100% |
| Stage #1: 2,2-dimethyl-cyclohexanone With lithium diisopropyl amide In tetrahydrofuran at -78℃; Stage #2: N,N-phenylbistrifluoromethane-sulfonimide In tetrahydrofuran at -78 - 23℃; | 100% |
| Stage #1: 2,2-dimethyl-cyclohexanone With n-butyllithium; diisopropylamine In tetrahydrofuran at -78℃; for 1h; Inert atmosphere; Stage #2: N,N-phenylbistrifluoromethane-sulfonimide In tetrahydrofuran at -78 - 20℃; for 2.5h; Inert atmosphere; | 55% |
| With lithium diisopropyl amide 1.) THF, -78 deg C, 2 h, 2.) THF, 0 deg C, 12 h; Yield given. Multistep reaction; |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
188823-98-5
raloxifene

-
-
188824-03-5
Trifluoro-methanesulfonic acid 6-[4-(2-piperidin-1-yl-ethoxy)-phenyl]-3-trifluoromethanesulfonyloxy-6H-5-oxa-11-thia-benzo[a]fluoren-9-yl ester

| Conditions | Yield |
|---|---|
| With triethylamine; N,N-dimethyl-formamide In tetrahydrofuran for 12h; | 100% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
214413-28-2
(4aS,5R,6R,8aS)-5,6,8a-Trimethyl-5-(2-triisopropylsilanyloxy-ethyl)-octahydro-naphthalen-1-one

-
-
218453-51-1
Trifluoro-methanesulfonic acid (4aS,5R,6R,8aS)-5,6,8a-trimethyl-5-(2-triisopropylsilanyloxy-ethyl)-3,4,4a,5,6,7,8,8a-octahydro-naphthalen-1-yl ester

| Conditions | Yield |
|---|---|
| With sodium hexamethyldisilazane In tetrahydrofuran at -78℃; for 1h; Esterification; | 100% |
| With lithium diisopropyl amide Yield given. Multistep reaction; |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
263160-77-6
dimethyl 5-iodo-2-{[(trifluoromethyl)sulphonyl]oxy}isophthalate

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 16h; sulphonylation; | 100% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
2423-71-4
2,6-dimethyl-4-nitro phenol

-
-
156740-78-2
2,6-dimethyl-4-nitrophenyl trifluoromethanesulphonate

| Conditions | Yield |
|---|---|
| With sodium hydride In DMF (N,N-dimethyl-formamide) at 0℃; for 2h; | 100% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 8h; Acylation; | 89% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
713-95-1
5-dodecanolide

-
-
445462-86-2
trifluoro-methanesulfonic acid 6-heptyl-5,6-dihydro-4H-pyran-2-yl ester

| Conditions | Yield |
|---|---|
| With potassium hexamethylsilazane In tetrahydrofuran; toluene at -78℃; for 0.25h; | 100% |
| With potassium hexamethylsilazane In tetrahydrofuran; toluene at -78℃; for 0.25h; |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
3164-07-6
4-(benzo[d]oxazol-2ʹ-yl)-2-methoxyphenol

-
-
540497-27-6
4-(1,3-benzoxazol-2-yl)-2-methoxyphenyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 20℃; for 0.5h; | 100% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
149105-13-5
4-acetyl-2-methoxyphenyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide; acetonitrile at 20℃; for 12h; | 100% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
6836-19-7
7-Methoxy-1-tetralone

-
-
724707-85-1
trifluoromethanesulfonic acid 7-methoxy-3,4-dihydronaphthalen-1-yl ester

| Conditions | Yield |
|---|---|
| Stage #1: 7-Methoxy-1-tetralone With lithium hexamethyldisilazane In tetrahydrofuran at -79℃; for 0.75h; Stage #2: N,N-phenylbistrifluoromethane-sulfonimide In tetrahydrofuran at -78 - 0℃; for 3h; | 100% |
| With potassium hexamethylsilazane In tetrahydrofuran at -78℃; for 1h; | 96% |
| With potassium hexamethylsilazane In tetrahydrofuran at -78℃; for 1.5h; | 96% |
-
-
4746-97-8
cyclohexanedione monoethylene ketal

-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
170011-47-9
1,4-dioxaspiro[4.5]dec-7-en-8-yl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With potassium hexamethylsilazane In tetrahydrofuran; toluene at -78℃; for 4h; | 100% |
| With lithium hexamethyldisilazane In tetrahydrofuran at -78 - 20℃; for 0.5h; Inert atmosphere; | 100% |
| With lithium hexamethyldisilazane at -78 - 20℃; Inert atmosphere; | 100% |
-
-
497-38-1
Norbornan-2-on

-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
82361-91-9
2-trifluoromethanesulfonyloxybicyclo[2.2.1]hept-2-ene

| Conditions | Yield |
|---|---|
| Stage #1: Norbornan-2-on With lithium diisopropyl amide In tetrahydrofuran at -78 - 20℃; for 0.5h; Inert atmosphere; Stage #2: N,N-phenylbistrifluoromethane-sulfonimide In tetrahydrofuran at -78 - 20℃; for 3h; Inert atmosphere; | 100% |
| With n-butyllithium; diisopropylamine In tetrahydrofuran | 59% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
207989-87-5
5-(4-hydroxy-phenyl)-pyrrolidin-2-one

-
-
207989-88-6
γ-4-trifluoromethylsulphonyloxyphenyl-γ-butyrolactam

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; | 100% |
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; | 96% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 25℃; for 12h; | 100% |
| With triethylamine In dichlorometane at 0 - 25℃; for 12h; | 100% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
1000289-72-4
1-[7-(5-cyanopyridin-2-yl)-5-hydroxybenzothiazol-2-yl]-3-ethylurea

-
-
1000289-73-5
trifluoromethanesulfonic acid 7-(5-cyanopyridin-2-yl)-2-(3-ethylureido)benzothiazol-5-yl ester

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 20℃; for 2h; | 100% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
958457-26-6
3-hydroxy-6-methyl-2-(O-tert-butyldimethylsilyl)hydroxymethylpyran-4(1H)-one

-
-
958457-27-7
6-methyl-2-(O-tert-butyldimethylsilyl)hydroxymethyl-3-trifluoromethanesulfonyloxy-pyran-4(1H)-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 1.5h; Inert atmosphere; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | |
| With potassium carbonate In N,N-dimethyl-formamide for 1.66667h; |

| Conditions | Yield |
|---|---|
| With 2-tert-butylimino-2-diethylamino-1,3-dimethyl-perhydro-1,3,2-diazaphosphorine In tetrahydrofuran at 20℃; | 100% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
693818-37-0
2-fluoro-5-(8-fluoro-7-trifluoromethylquinolin-4-yl)phenol

-
-
693818-38-1
trifluoromethanesulphonic acid 2-fluoro-5-(8-fluoro-7-trifluoromethyl-quinolin-4-yl)phenyl ester

| Conditions | Yield |
|---|---|
| With triethylamine at 20℃; for 16h; | 100% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
54815-88-2
methyl 1-hydroxy-5,6,7,8-tetrahydronaphthalene-2-carboxylate

-
-
812690-21-4
methyl 1-{[(trifluoromethyl)sulphonyl]oxy}-5,6,7,8-tetrahydronaphthalene-2-carboxylate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; dmap In dichloromethane at 20℃; for 18.5h; | 100% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
762300-05-0
C29H40N2O4

-
-
762300-07-2
C30H39F3N2O6S

| Conditions | Yield |
|---|---|
| Stage #1: N,N-phenylbistrifluoromethane-sulfonimide; C29H40N2O4 With triethylamine In dichloromethane at 20℃; Stage #2: With sodium hydroxide In dichloromethane; water at 20℃; for 0.5h; | 100% |
-
-
74668-72-7
2-methoxy-8-hydroxyquinoline

-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
642477-88-1
1,1,1-trifluoromethanesulfonic acid 2-methoxyquinolin-8-yl ester

| Conditions | Yield |
|---|---|
| With triethylamine In DMF (N,N-dimethyl-formamide) at 40℃; for 8h; | 100% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 100% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
911227-82-2
tert-butyl 5-(methoxymethoxy)-4-(trifluoromethylsulfonyloxy)spiro[chromene-2,4'-piperidine]-1'-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl 5-(methoxymethoxy)-4-oxospiro[chroman-2,4'-piperidine]-1'-carboxylate With lithium hexamethyldisilazane In tetrahydrofuran at -78℃; for 1h; Stage #2: N,N-phenylbistrifluoromethane-sulfonimide In tetrahydrofuran at -78 - 20℃; for 12h; | 100% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
911228-44-9
C23H22F3NO5S

| Conditions | Yield |
|---|---|
| Stage #1: benzyl 4-oxo-3,4-dihydro-1H-spiro[naphthalene-2,4'-piperidine]-1'-carboxylate With lithium hexamethyldisilazane In tetrahydrofuran at -78℃; for 0.75h; Stage #2: N,N-phenylbistrifluoromethane-sulfonimide In tetrahydrofuran at -78 - 20℃; for 2.5h; | 100% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
852204-40-1
(3R,4S)-3-[(3S)-3-{[tert-butyl(dimethyl)silyl]oxy}-3-(4-fluorophenyl)propyl]-4-(3'-hydroxybiphenyl-4-yl)-1-phenylazetidin-2-one

-
-
852204-41-2
4'-{(2S,3R)-3-[(3S)-3-{[tert-butyl(dimethyl)silyl]oxy}-3-(4-fluorophenyl)propyl]-4-oxo-1-phenylazetidin-2-yl}biphenyl-3-yl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 20℃; for 2h; | 100% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
920511-31-5
2-({4-[(4-fluorophenyl)amino]-1-piperidinyl}carbonyl)-3-pyridinol

-
-
920511-33-7
2-({4-[(4-fluorophenyl)amino]-1-piperidinyl}carbonyl)-3-pyridinyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 100% |
| With triethylamine In dichloromethane at 20℃; | |
| With triethylamine In dichloromethane at 20℃; |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide


| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide | 100% |
N,N-Bis(trifluoromethylsulfonyl)aniline Chemical Properties
Product Name: N-Phenylbis(trifluoromethanesulphonimide) (CAS NO.37595-74-7)
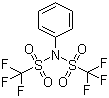
Molecular Formula: C8H5F6NO4S2
Molecular Weight: 357.25g/mol
Mol File: 37595-74-7.mol
Appearance: white to off-white crystalline powder
Melting Point: 100-102 °C
Boiling point: 305.3 °C at 760 mmHg
Flash Point: 138.4 °C
Density: 1.766 g/cm3
Surface Tension: 44.6 dyne/cm
Enthalpy of Vaporization: 54.57 kJ/mol
Vapour Pressure: .000828 mmHg at 25°C
XLogP3-AA: 3.1
H-Bond Donor: 0
H-Bond Acceptor: 11
Structure Descriptors of N-Phenylbis(trifluoromethanesulphonimide) (CAS NO.37595-74-7):
IUPAC Name: 1,1,1-trifluoro-N-phenyl-N-(trifluoromethylsulfonyl)methanesulfonamide
Canonical SMILES: C1=CC=C(C=C1)N(S(=O)(=O)C(F)(F)F)S(=O)(=O)C(F)(F)F
InChI: InChI=1S/C8H5F6NO4S2/c9-7(10,11)20(16,17)15(6-4-2-1-3-5-6)21(18,19)8(12,13)14/h1-5H
InChIKey: DIOHEXPTUTVCNX-UHFFFAOYSA-N
Product Categories: API intermediates
N,N-Bis(trifluoromethylsulfonyl)aniline Safety Profile
Safety Information of N-Phenylbis(trifluoromethanesulphonimide) (CAS NO.37595-74-7):
Hazard Codes: Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
WGK Germany: 3
F: 21
Hazard Note: Irritant
HazardClass: IRRITANT
N,N-Bis(trifluoromethylsulfonyl)aniline Specification
N-Phenylbis(trifluoromethanesulphonimide) , its CAS NO. is 37595-74-7, the synonyms are 1,1,1-Trifluoro-N-phenyl-N-[(trifluoromethyl)sulfonyl]methanesulfonamide ; N,N-Bis(trifluoromethylsulfonyl)aniline .
Related Products
- N-[(10-Oxido-9,10-dihydro-9-oxa-10-phosphaphenanthrene)methyl]-1,3,5-triazine-2,4,6-triamine
- N10-(Trifluoroacetyl)pteroic acid
- N-[1,1'-Biphenyl]-4-yl-9,9-dimethyl-9H-fluoren-2-amine
- N-[1,1'-Biphenyl]-4-yl-9,9-dimethyl-9H-fluoren-3-amine
- N-[1,1'-Biphenyl]-4-yl-N-(4-bromophenyl)-9,9-dimethyl-9H-fluoren-2-amine
- N-[1,1-Bis[(acetyloxy)methyl]-3-(4-octylphenyl)propyl]acetamide
- N'-[(1,1-Dimethylethoxy)carbonyl]-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-N'-methyl-L-lysine
- N1,1-Diphenyl-1,2-ethanediamine
- N-(1,2-Dimethylpropyl)-2-pyridinamine
- N<sup xmlns="">1</sup>-(3,4-DIMETHYL-5-ISOXAZOLYL)SULFANIL-AMIDE LITHIUM SALT
- 375-96-2
- 37597-96-9
- 37599-14-7
- 3759-92-0
- 37599-58-9
- 37599-83-0
- 3760-11-0
- 376-03-4
- 37-60-5
- 3760-52-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View