-
Name
CAPRYLOHYDROXAMIC ACID
- EINECS 230-936-7
- CAS No. 7377-03-9
- Article Data30
- CAS DataBase
- Density 0.97 g/cm3
- Solubility
- Melting Point 78 °C
- Formula C8H17NO2
- Boiling Point
- Molecular Weight 159.228
- Flash Point
- Transport Information
- Appearance
- Safety 26-36/37/39
- Risk Codes 36/37/38
-
Molecular Structure
- Hazard Symbols
- Synonyms Octanohydroxamicacid (6CI,7CI,8CI);Caprylohydroxamic acid;N-Hydroxyoctanamide;Octanoylhydroxamic acid;Taselin;
- PSA 49.33000
- LogP 2.24320
Synthetic route

-
-
7377-03-9
caprylohydroxamic acid

| Conditions | Yield |
|---|---|
| With Novozym 435 (Candida antarctica lipase B on Lewatit E); hydroxylamine In water at 40℃; for 20h; Condensation; Enzymatic reaction; | 99% |
| Stage #1: Octanoic acid With acetic anhydride for 0.166667h; Stage #2: With hydroxylamine hydrochloride for 0.666667h; | 95.81% |
| With liverextract; hydroxylamine | |
| With hydroxylamine; 1,1'-carbonyldiimidazole | |
| With hydroxylamine; adenosine monophosphate ligase SfaB from Streptomyces thioluteus; ATP; magnesium chloride; Cleland's reagent In aq. buffer at 30℃; for 6h; pH=8; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| Stage #1: octanoic acid ethyl ester With sodium ethanolate; sodium carbonate In ethanol at 40℃; for 2.5h; Stage #2: With hydroxylamine In ethanol; water | 95.7% |
| With sodium sulfide; hydroxylamine hydrochloride; sodium hydroxide In ethanol at 20 - 45℃; for 3h; Temperature; Concentration; | 93.1% |
| With hydroxylamine hydrochloride; potassium hydroxide In ethanol; water at 5 - 55℃; for 3h; Concentration; Temperature; | 91.1% |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium hydroxide In methanol; water at 5 - 40℃; for 5h; Concentration; Temperature; Large scale; | 94.7% |
| With hydroxylamine hydrochloride; sodium hydroxide at 30℃; for 6h; pH=13; | 92.3% |
| With hydroxylamine hydrochloride; triethylamine In water at 0 - 50℃; for 14.5h; Temperature; | 85% |

| Conditions | Yield |
|---|---|
| With methylamine In methanol for 2h; Irradiation; | 85% |
| With selenium(IV) oxide; triethylamine In dichloromethane 1.) 0-10 deg C, 10 min. 2.) 20 deg C, 30 min. 3.) reflux, 1h; | 76% |
| With sodium hydroxide; ethanol for 6h; Product distribution; Quantum yield; Irradiation; var. reagents and solvents; other nitroalkanes; | 30% |
| With sodium ethanolate In ethanol Irradiation; | 30% |
| With sodium ethanolate In ethanol Quantum yield; Irradiation; |

| Conditions | Yield |
|---|---|
| With hydroxylamine nitrate; sodium hydroxide In methanol at 0 - 50℃; for 3h; | A 84% B 3 g |

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide; triethylamine In dichloromethane Product distribution; Mechanism; various molar ratio of reagents; also in the absence of triethylamine; | A 15% B 50% |

| Conditions | Yield |
|---|---|
| With air; tris-trimethylsilyl-hydroxylamine 1.) hexane, RT, 5 min, 2.) 1 d; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With Thermomyces lanuginosus lipase on Accurel EP; water; hydroxylamine In tert-butyl alcohol at 40℃; for 96h; Kinetics; Condensation; hydrolysis; Enzymatic reaction; |
-
-
71478-41-6
(ethoxycarbonyl)octanoate

-
-
7377-03-9
caprylohydroxamic acid

| Conditions | Yield |
|---|---|
| With hydroxylamine In methanol; diethyl ether at 20℃; for 0.25h; | 2.92 g |

-
-
7377-03-9
caprylohydroxamic acid

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; triethylamine In dichloromethane at 20℃; for 20h; |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 0.5h; | 99% |
| In dichloromethane at 20℃; for 0.5h; | 70% |
| In dichloromethane at 20℃; for 0.5h; |
-
-
7377-03-9
caprylohydroxamic acid

-
-
75-65-0
tert-butyl alcohol

-
-
38427-89-3
(N-tert-Butyloxycarbonyl)heptylamine

| Conditions | Yield |
|---|---|
| Stage #1: caprylohydroxamic acid With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; triethylamine In ethyl acetate at 0℃; for 3h; Stage #2: tert-butyl alcohol In ethyl acetate at 60℃; for 1h; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: caprylohydroxamic acid With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; triethylamine In ethyl acetate at 0℃; for 3h; Stage #2: benzyl alcohol In ethyl acetate at 60℃; for 1h; | 96% |
-
-
7704-67-8
erythromycin A thiocyanate

-
-
7377-03-9
caprylohydroxamic acid

-
-
342371-84-0
9-deoxo-6-deoxy-6,9-epoxy-9,9a-didehydro-9a-aza-homoerythromycin A

| Conditions | Yield |
|---|---|
| Stage #1: erythromycin thiocyanate A; caprylohydroxamic acid In acetonitrile at 20℃; for 0.166667h; Stage #2: With sulfuric acid In dimethyl sulfoxide; acetone at 80℃; for 16h; Concentration; Temperature; Reagent/catalyst; Solvent; | 90% |
-
-
7377-03-9
caprylohydroxamic acid

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
917470-39-4
O-tert-butyldimethylsilyl-heptanohydroxamic acid

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 16h; | 84% |

| Conditions | Yield |
|---|---|
| In acetonitrile for 15h; Ambient temperature; | 69% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 56.1% |
-
-
530-48-3
1,1-Diphenylethylene

-
-
7377-03-9
caprylohydroxamic acid

-
-
145838-86-4
bis(methoxycarbonyl)(phenyliodinio)methanide

| Conditions | Yield |
|---|---|
| In toluene at 70℃; Inert atmosphere; Darkness; | 46% |

-
-
7377-03-9
caprylohydroxamic acid

-
-
108-24-7
acetic anhydride

-
-
29264-59-3
N-Octanoyl-O-acetylhydroxylamin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 69 percent / acetonitrile / 15 h / Ambient temperature 2: 91 percent / 120 °C View Scheme |

-
-
7377-03-9
caprylohydroxamic acid

| Conditions | Yield |
|---|---|
| In ethanol; water treating of an aqueous soln. of UO2(NO3)2 with the hydroxamic acid in aqueous ethanol, standing on a steam bath for 30 min; filtn., washing (water, ether), drying, elem. anal.; |
-
-
7377-03-9
caprylohydroxamic acid

| Conditions | Yield |
|---|---|
| With magnesium(II) perchlorate In chloroform using bulk liq. membrane transport from aq. source phase through CHCl3 membrane phase to aq. receiving phase; source phase: aq. Mg(ClO4)2, Fe complex, pH 5.0; membrane phase: ligand in CHCl3; receiving phase: aq. Mg(ClO4)2, pH 0.5-3.5; monitored by UV-vis spectra; |
-
-
7377-03-9
caprylohydroxamic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dichloromethane / 0.5 h / 20 °C 2: silver hexafluoroantimonate; di-μ-chloro-bis[chloro(η5-pentamethylcyclopentadienyl)cobalt] / 1,2-dichloro-ethane / 24 h / 80 °C / 760.05 Torr View Scheme |

| Conditions | Yield |
|---|---|
| With water; cetyltrimethylammonim bromide; butan-1-ol In n-heptane at 26.84℃; pH=9.2; Kinetics; pH-value; Reagent/catalyst; Solvent; |


| Conditions | Yield |
|---|---|
| With dodecyltrimethylammonium bromide In aq. buffer at 26.84℃; pH=9.2; Reagent/catalyst; |
-
-
7377-03-9
caprylohydroxamic acid

-
-
10359-36-1
4-nitro-phenyl diphenyl phosphate

-
B
-
14609-74-6
p-nitrophenolate

| Conditions | Yield |
|---|---|
| With potassium chloride; N,N-didodecyl-N,N-dimethylammonium bromide In aq. buffer at 26.84℃; pH=9.2; Kinetics; Concentration; pH-value; Reagent/catalyst; |

-
-
7377-03-9
caprylohydroxamic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dichloromethane / 0.5 h / 20 °C 2: carbonyl(pentamethylcyclopentadienyl)cobalt diiodide; silver(I) triflimide; zinc diacetate / 1,2-dichloro-ethane / 120 °C View Scheme |
-
-
7377-03-9
caprylohydroxamic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dichloromethane / 0.5 h / 20 °C / Inert atmosphere 2: Cp*Co(CO)I2; silver hexafluoroantimonate; (adamant-1-yl)-acetic acid / chlorobenzene / 12 h / 100 °C / Inert atmosphere; Sealed tube View Scheme |
Octanamide, N-hydroxy- Specification
The CAS register number of Octanamide, N-hydroxy- is 7377-03-9. It also can be called as Octanoylhydroxamic acid and the IUPAC name about this chemical is N-hydroxyoctanamide. The molecular formula about this chemical is C8H17NO2 and the molecular weight is 159.23. It belongs to the following product categories which include Hydroxylamines; Hydroxylamines (N-Substituted) and so on.
Physical properties about Octanamide, N-hydroxy- are: (1)ACD/LogP: 1.60; (2)ACD/LogD (pH 5.5): 1.6; (3)ACD/LogD (pH 7.4): 1.6; (4)ACD/BCF (pH 5.5): 9.65; (5)ACD/BCF (pH 7.4): 9.58; (6)ACD/KOC (pH 5.5): 176.33; (7)ACD/KOC (pH 7.4): 175.14; (8)#H bond acceptors: 3; (9)#H bond donors: 2; (10)#Freely Rotating Bonds: 7; (11)Polar Surface Area: 29.54 Å2; (12)Index of Refraction: 1.452; (13)Molar Refractivity: 44.27 cm3; (14)Molar Volume: 164 cm3; (15)Polarizability: 17.55x10-24cm3; (16)Surface Tension: 35.4 dyne/cm; (17)Density: 0.97 g/cm3.
Preparation: this chemical can be prepared by octanoic acid. This reaction will need reagents of liverextract, hydroxylamine.

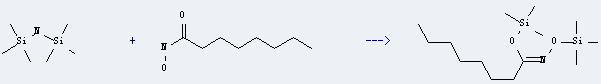
Uses of Octanamide, N-hydroxy-: it can be used to produce C14H33NO2Si2 with 1,1,1,3,3,3-hexamethyl-disilazane. This reaction will need solvent of acetonitrile. The reaction time is 15 hours with ambient temperature. The yield is about 69%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. If you want to use this chemical, wear suitable protective clothing, gloves and eye/face protection. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. If you want to store it, you should keep the container tightly sealed in dry, cool places. If you store and use this chemical according the rule, it will not be decomposed.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(NO)CCCCCCC
(2)InChI: InChI=1/C8H17NO2/c1-2-3-4-5-6-7-8(10)9-11/h11H,2-7H2,1H3,(H,9,10)
(3)InChIKey: RGUVUPQQFXCJFC-UHFFFAOYAM
(4)Std. InChI: InChI=1S/C8H17NO2/c1-2-3-4-5-6-7-8(10)9-11/h11H,2-7H2,1H3,(H,9,10)
(5)Std. InChIKey: RGUVUPQQFXCJFC-UHFFFAOYSA-N
The toxicity data are as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | > 800mg/kg (800mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 196, Pg. 478, 1976. | |
| mouse | LD50 | oral | 8820mg/kg (8820mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 42(3), Pg. 99, 1977. | |
| rat | LD50 | oral | 10700mg/kg (10700mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 42(3), Pg. 99, 1977. |
Related Products
- Octanamide, N-hydroxy-
- 7377-08-4
- 73771-04-7
- 73771-11-6
- 737713-28-9
- 73771-62-7
- 737718-34-2
- 73772-38-0
- 7377-26-6
- 737751-95-0
- 737760-98-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View