-
Name
Phenyl salicylate
- EINECS 204-259-2
- CAS No. 118-55-8
- Article Data77
- CAS DataBase
- Density 1.25 g/cm3
- Solubility Water: 1 g/6670 mL
- Melting Point 41.5 °C
- Formula C13H10O3
- Boiling Point 306.6 °C at 760 mmHg
- Molecular Weight 214.221
- Flash Point 137.3 °C
- Transport Information
- Appearance White crystalline solid
- Safety 26-36-24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Benzoic acid,2-hydroxy-,esters,phenyl ester;Benzoic acid, 2-hydroxy-, phenyl ester;2-Hydroxybenzoic acid, phenyl ester;Phenyl-2-hydroxybenzoate;Fenylester kyseliny salicylove;Phenylsalicylate;2-Phenoxycarbonylphenol;Phenol salicylate;Musol;Salicylic acid, phenyl ester;Salphenyl;Fenylester kyseliny salicylove [Czech];
- PSA 46.53000
- LogP 2.61140
Synthetic route
-
-
116577-48-1
2-(4-Oxo-pentanoyloxy)-benzoic acid phenyl ester

-
-
118-55-8
phenyl Salicylate

| Conditions | Yield |
|---|---|
| With sodium metabisulfite; sodium thiosulfate In tetrahydrofuran; water for 0.5h; Product distribution; Ambient temperature; deprotection; | 93% |

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; Lumogen F Orange 240 In acetonitrile at 40℃; for 26h; Catalytic behavior; Reagent/catalyst; Solvent; Wavelength; Irradiation; Inert atmosphere; | 92% |
| With sodium carbonate; 9-(2-mesityl)-10-methylacridinium perchlorate In water; acetonitrile at 20℃; for 16h; Quantum yield; Catalytic behavior; Solvent; Concentration; Time; Smiles Aromatic Rearrangement; Irradiation; | 75% |
| With dipotassium peroxodisulfate; potassium trifluoroacetate; silver nitrate In acetonitrile at 130℃; for 36h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Smiles Aromatic Rearrangement; Sealed tube; Inert atmosphere; | 64% |
-
-
17954-26-6
1,2-dihydro-2-phenoxycarbonylisoquinoline-1-carbonitrile

-
-
69-72-7
salicylic acid

-
-
118-55-8
phenyl Salicylate

| Conditions | Yield |
|---|---|
| at 135℃; for 2.5h; | 86% |

| Conditions | Yield |
|---|---|
| With aluminium trichloride; zinc(II) chloride at 0 - 20℃; for 10h; | 85% |
| With trichlorophosphate at 75 - 80℃; for 4h; | 70% |
| With trichlorophosphate |

| Conditions | Yield |
|---|---|
| With 6% Mo(VI)/ZrO2 coated on сordierite honeycomb monolith for 4h; Reflux; | 85% |
| With sodium | |
| With poisoned SO42-/ZrO2 at 250℃; for 1h; | 36.6 %Chromat. |
| Reflux; |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; 1,10-Phenanthroline; sodium carbonate In 1,4-dioxane at 95℃; for 17h; Reagent/catalyst; Solvent; Temperature; Molecular sieve; Green chemistry; | 85% |

| Conditions | Yield |
|---|---|
| With rhodium(III) chloride; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; 1,2-bis-(dicyclohexylphosphino)ethane In 1,4-dioxane; toluene at 120℃; under 4500.45 Torr; for 24h; Catalytic behavior; Reagent/catalyst; Pressure; Molecular sieve; Autoclave; | 81% |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 75 - 145℃; for 4h; Temperature; | 74% |

| Conditions | Yield |
|---|---|
| With carbon dioxide; n-butylstannoic acid; di(n-butyl)tin oxide at 220℃; under 3750.38 Torr; for 3h; Reagent/catalyst; Pressure; Temperature; | 66.9% |

| Conditions | Yield |
|---|---|
| With rhodium(III) chloride; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; 1,2-bis-(dicyclohexylphosphino)ethane In 1,4-dioxane; toluene at 120℃; under 4500.45 Torr; for 24h; Molecular sieve; Autoclave; | 65% |

-
-
118-55-8
phenyl Salicylate

| Conditions | Yield |
|---|---|
| With iodine; bis-[(trifluoroacetoxy)iodo]benzene In dichloromethane; benzene at 60 - 70℃; for 2h; Irradiation; | 54% |

| Conditions | Yield |
|---|---|
| With n-butylstannoic acid at 220℃; under 3750.38 Torr; for 8h; Reagent/catalyst; Pressure; Temperature; | A 31% B 53% C 7% |


| Conditions | Yield |
|---|---|
| With copper(II) bis(trifluoromethanesulfonate); urea In ethyl acetate at 60℃; for 12h; Chan-Lam reaction; | 51% |

| Conditions | Yield |
|---|---|
| With Trichlorbutylstannan at 220℃; under 3750.38 Torr; for 8h; | A 24% B 45% |
-
-
124-38-9
carbon dioxide

-
-
108-95-2
phenol

-
A
-
102-09-0
bis(phenyl) carbonate

-
B
-
118-55-8
phenyl Salicylate

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; potassium carbonate; zinc(II) chloride In tetrachloromethane at 120℃; under 22501.8 Torr; for 6h; | A 29.7% B 2.2% |
| With tetrachloromethane; potassium carbonate at 120℃; under 7500.75 Torr; for 6h; | A 74 %Chromat. B 10.7 %Chromat. |
| With tetrachloromethane; potassium carbonate at 120℃; under 7500.75 Torr; for 6h; | A 40.8 %Chromat. B 59.2 %Chromat. |
| With C72H76N2O4P2Ti(2+)*2Cl(1-); potassium carbonate In methanol at 100℃; under 45004.5 Torr; for 8h; Catalytic behavior; Reagent/catalyst; Pressure; Temperature; Autoclave; | A 2.88 mmol B 1.18 mmol |

-
-
124-38-9
carbon dioxide

-
-
100-67-4
potassium phenolate

-
A
-
102-09-0
bis(phenyl) carbonate

-
B
-
118-55-8
phenyl Salicylate

| Conditions | Yield |
|---|---|
| With tetramethylammonium bromide; zinc(II) chloride In tetrachloromethane at 120℃; under 22501.8 Torr; for 6h; | A 2.9% B 23% |

-
-
205178-61-6
2-Trifluoromethanesulfonyloxy-benzoic acid phenyl ester

-
A
-
2005-10-9
6H-benzo[c]chromen-6-one

-
B
-
118-55-8
phenyl Salicylate

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; sodium pivalate In N,N-dimethyl acetamide at 80℃; for 10h; Heating; | A 22% B 21% |


| Conditions | Yield |
|---|---|
| durch Erhitzen im Phosgenstrom; | |
| durch Erhitzen im Phosgenstrom; |

| Conditions | Yield |
|---|---|
| With sulfuric acid | |
| With hydrogenchloride |
-
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

-
-
69-72-7
salicylic acid

-
-
118-55-8
phenyl Salicylate

| Conditions | Yield |
|---|---|
| at 230℃; Destillation des entstehenden Wassers; |

| Conditions | Yield |
|---|---|
| at 210 - 230℃; | |
| at 250 - 350℃; | |
| With aluminium trichloride; thionyl chloride Behandeln des Reaktionsprodukts mit Phenol oder mit wss. Natriumphenolat-Loesung; |

| Conditions | Yield |
|---|---|
| at 200 - 220℃; |
-
-
69-72-7
salicylic acid

-
A
-
118-55-8
phenyl Salicylate

-
B
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

-
C
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| at 250 - 350℃; |


| Conditions | Yield |
|---|---|
| With PPA |

| Conditions | Yield |
|---|---|
| Reaktion von Natriumphenolat; | |
| Reaktion von Natriumphenolat; |


| Conditions | Yield |
|---|---|
| With 10 percent human plasma In acetonitrile at 37℃; Rate constant; phosphate buffer (pH 7.4); or in undiluted plasma; | |
| With phosphate buffer; hydroxide In 1,4-dioxane; water at 37℃; Rate constant; |
-
-
56-23-5
tetrachloromethane

-
-
108-95-2
phenol

-
A
-
34888-05-6
(trichloromethoxy)benzene

-
B
-
17696-62-7
Phenyl 4-hydroxybenzoate

-
C
-
118-55-8
phenyl Salicylate

-
D
-
1441-87-8
salicyloyl chloride

| Conditions | Yield |
|---|---|
| for 5h; Irradiation; Yield given. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| at 200℃; |
-
-
118-55-8
phenyl Salicylate

-
-
107-15-3
ethylenediamine

-
-
6345-72-8
N,N'-bis(2-hydroxybenzoyl)ethylenediamine

| Conditions | Yield |
|---|---|
| at 180 - 190℃; for 1h; | 100% |
| at 125℃; for 1.5h; | 80% |
| With triethylamine In isopropyl alcohol at 80℃; for 0.5h; | 67% |
| With 1,2,4-Trichlorobenzene |
-
-
16837-38-0
nicotinic anhydride

-
-
118-55-8
phenyl Salicylate

-
-
121985-90-8
2-nicotinoyloxy-benzoic acid phenyl ester

| Conditions | Yield |
|---|---|
| With pyridine | 99% |
-
-
118-55-8
phenyl Salicylate

-
-
111-36-4
n-butyl isocyanide

-
A
-
3898-47-3
phenyl-N-butylcarbamate

-
B
-
162936-60-9
3-butyl-3,4-dihydro-2H-1,3-benzoxazine-2,4-dione

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dimethyl sulfoxide for 12h; Ambient temperature; | A n/a B 98% |


| Conditions | Yield |
|---|---|
| at 20 - 162℃; for 0.0833333h; microwave irradiation; | 98% |
-
-
118-55-8
phenyl Salicylate

-
-
3433-80-5
1-Bromo-2-bromomethyl-benzene

-
-
1380084-70-7
2-(2-bromobenzyloxy)benzoic acid phenyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Inert atmosphere; Reflux; | 98% |
-
-
129368-70-3
(6-bromohexyloxy)-tert-butyldimethylsilane

-
-
118-55-8
phenyl Salicylate

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; nickel(II) bromide 2-methoxyethyl ether complex; zinc In N,N-dimethyl-formamide at 60℃; for 10h; Inert atmosphere; Sealed tube; | 98% |
-
-
118-55-8
phenyl Salicylate

-
-
3173-53-3
Cyclohexyl isocyanate

-
A
-
3417-55-8
3-cyclohexyl-benzo[e][1,3]oxazine-2,4-dione

-
B
-
56379-88-5
cyclohexylcarbamic acid phenyl ester

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In N,N-dimethyl-formamide for 72h; Ambient temperature; | A 97% B n/a |

-
-
118-55-8
phenyl Salicylate

-
-
1795-48-8
Isopropyl isocyanate

-
A
-
17614-10-7
phenyl N-isopropylcarbamate

-
B
-
159977-54-5
3-Isopropyl-benzo[e][1,3]oxazine-2,4-dione

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In N,N-dimethyl-formamide for 45h; Ambient temperature; | A n/a B 97% |

-
-
54208-70-7
N-(1,3-dimethylbutyl)-p-phenylenediamine

-
-
118-55-8
phenyl Salicylate

| Conditions | Yield |
|---|---|
| at 20 - 201℃; for 0.0833333h; microwave irradiation; | 97% |

| Conditions | Yield |
|---|---|
| at 20 - 205℃; for 0.0666667h; microwave irradiation; | 96% |

| Conditions | Yield |
|---|---|
| With iodine; triethylamine; triphenylphosphine In dichloromethane at 0 - 20℃; for 0.333333h; | 96% |

| Conditions | Yield |
|---|---|
| at 20 - 157℃; for 0.133333h; microwave irradiation; | 95% |
| With boron trifluoride diethyl etherate In toluene at 20℃; | 76% |
| at 200℃; |

| Conditions | Yield |
|---|---|
| at 20 - 171℃; for 0.0666667h; microwave irradiation; | 95% |
| at 180 - 200℃; for 3h; | 70% |

| Conditions | Yield |
|---|---|
| at 200℃; for 4h; | 94.1% |
-
-
118-55-8
phenyl Salicylate

-
-
101-54-2
N-phenylphenylene-1,4-diamine

-
-
30313-55-4
2-hydroxy-N-(4-(phenylamino)phenyl)benzamide

| Conditions | Yield |
|---|---|
| at 20 - 186℃; for 0.0833333h; microwave irradiation; | 93% |

| Conditions | Yield |
|---|---|
| With racemic-2-(di-tert-butylphosphino)-1,1′-binaphthyl; (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; cyclo-octa-1,5-diene at 170℃; for 20h; Kinetics; Reagent/catalyst; Temperature; Friedel-Crafts Acylation; Inert atmosphere; Sealed tube; regioselective reaction; | 93% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; water at 20℃; for 12h; | 92% |
| With 2,2'-azobis(isobutyronitrile); bis(tri-n-butyltin)oxide In benzene at 80℃; for 2.5h; | 73% |
| With sodium hydroxide In water; tert-butyl alcohol at 30 - 55℃; Thermodynamic data; Mechanism; reactions in var. solvents, CH3CN, Me2SO), between var. conditions; ΔG(excit), ΔH(excit, ΔS(excit); | |
| With sodium hydroxide; polyoxyethylene 23 lauryl ether In water at 35℃; Kinetics; Further Variations:; Reaction partners; | |
| With ethanol; cetyltrimethylammonim bromide; sodium hydroxide In water at 31.84℃; Kinetics; Mechanism; Reagent/catalyst; Temperature; Micellar solution; |
-
-
118-55-8
phenyl Salicylate

-
-
106-50-3
1,4-phenylenediamine

-
A
-
3679-65-0
N-(4-aminophenyl)-2-hydroxybenzamide

-
B
-
20073-94-3
N,N'-1,4-phenylenebis(2-hydroxybenzamide)

| Conditions | Yield |
|---|---|
| at 20 - 210℃; for 0.116667h; microwave irradiation; | A 92% B 6% |

-
-
14273-90-6
methyl 6-bromohexanoate

-
-
118-55-8
phenyl Salicylate

-
-
133535-19-0
methyl 7-(2-hydroxyphenyl)-7-oxoheptanoate

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; nickel(II) bromide 2-methoxyethyl ether complex; zinc In N,N-dimethyl-formamide at 60℃; for 10h; Catalytic behavior; Reagent/catalyst; Temperature; Inert atmosphere; Sealed tube; | 92% |
-
-
118-55-8
phenyl Salicylate

-
-
2550-36-9
(bromomethylcyclohexane)

-
-
1414926-65-0
2-cyclohexyl-1-(2-hydroxyphenyl)ethan-1-one

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; nickel(II) bromide 2-methoxyethyl ether complex; zinc In N,N-dimethyl-formamide at 60℃; for 10h; Inert atmosphere; Sealed tube; | 92% |

| Conditions | Yield |
|---|---|
| at 200℃; for 4h; | 90.8% |
-
-
934-32-7
1H-benzimidazol-2-amine

-
-
118-55-8
phenyl Salicylate

-
-
61745-68-4
N-(benzimidazol-2-yl)salicylamide

| Conditions | Yield |
|---|---|
| at 190 - 210℃; for 1.5h; | 90% |
| 210 deg C, 1.5 h; ethanol, reflux, 5 min.; | 66% |
| at 210℃; for 1.5h; |
-
-
29927-08-0
2-amino-5,6-dimethylbenzothiazole

-
-
118-55-8
phenyl Salicylate

-
-
123199-79-1
N-(5,6-dimethylbenzothiazol-2-yl)salicylamide

| Conditions | Yield |
|---|---|
| 210 deg C, 1.5 h; ethanol, reflux, 5 min.; | 90% |
| at 210℃; for 1.5h; |
-
-
118-55-8
phenyl Salicylate

-
-
110-60-1
1,4-diaminobutane

-
-
76218-89-8
N,N'-(butane-1,4-diyl)bis(2-hydroxybenzamide)

| Conditions | Yield |
|---|---|
| at 180℃; for 1.5h; | 90% |
-
-
118-55-8
phenyl Salicylate

-
-
95-53-4
o-toluidine

-
A
-
7133-56-4
2-hydroxy-N-(2-methylphenyl)benzamide

-
B
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| With 1,2,4-Trichlorobenzene for 0.5h; Heating / reflux; | A 90% B n/a |

-
-
118-55-8
phenyl Salicylate

-
-
920-46-7
Methacryloyl chloride

-
-
33374-44-6
phenyl 2-(methacryloxy)benzoate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at -15 - 20℃; for 20h; Inert atmosphere; | 90% |
-
-
1647-26-3
1-bromo-2-cyclohexylethane

-
-
118-55-8
phenyl Salicylate

-
-
108975-02-6
3-cyclohexyl-1-(2-hydroxyphenyl)propan-1-one

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; nickel(II) bromide 2-methoxyethyl ether complex; zinc In N,N-dimethyl-formamide at 60℃; for 10h; Inert atmosphere; Sealed tube; | 90% |
-
-
118-55-8
phenyl Salicylate

-
-
172995-33-4
((6-bromohexyl)oxy)(tert-butyl)diphenylsilane

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; nickel(II) bromide 2-methoxyethyl ether complex; zinc In N,N-dimethyl-formamide at 60℃; for 10h; Inert atmosphere; Sealed tube; | 90% |
Phenyl salicylate Consensus Reports
Phenyl salicylate Specification
The Phenyl salicylate with CAS registry number of 118-55-8 is also known as 2-Phenoxycarbonylphenol. The IUPAC name is Phenyl 2-hydroxybenzoate. It belongs to product categories of Aromatic Esters; Functional Materials; Liquid Crystals & Related Compounds; Phenyl Esters (Liquid Crystals). Its EINECS registry number is 204-259-2. In addition, the formula is C13H10O3 and the molecular weight is 214.22. This chemical is a white crystalline solid and should be sealed in a ventilated, cool place away from light, fire and oxides.
Physical properties about Phenyl salicylate are: (1)ACD/LogP: 3.55; (2)ACD/LogD (pH 5.5): 3.55; (3)ACD/LogD (pH 7.4): 3.53; (4)ACD/BCF (pH 5.5): 295.48; (5)ACD/BCF (pH 7.4): 281.91; (6)ACD/KOC (pH 5.5): 2041.42; (7)ACD/KOC (pH 7.4): 1947.66; (8)#H bond acceptors: 3; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 4; (11)Index of Refraction: 1.615; (12)Molar Refractivity: 59.77 cm3; (13)Molar Volume: 171.2 cm3; (14)Surface Tension: 51.8 dyne/cm; (15)Density: 1.25 g/cm3; (16)Flash Point: 137.3 °C; (17)Enthalpy of Vaporization: 56.9 kJ/mol; (18)Boiling Point: 306.6 °C at 760 mmHg; (19)Vapour Pressure: 0.000421 mmHg at 25 °C.
Preparation of Phenyl salicylate: it is prepared by reaction of phenol with salicylic acid. Firstly, the raw material is added to reactor and heated to melt at 128-132 °C for 4 hours under stirring. Meanwhile, phosphorus trichloride is added to the reaction mixture slowly. At last, product is obtained by washing, cooling, crystallization and bleaching.
C7H6O3+C6H6O→C13H10O3+H2O
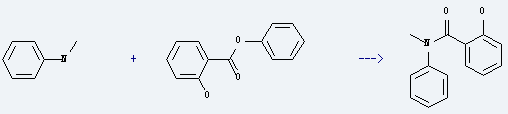
Uses of Phenyl salicylate: it is used to produce N-methyl-salicylanilide by reaction with N-methyl-aniline. The reaction occurs at the temperature of 180-200 for 3 hours. The yield is about 69%. What's more, it is used as UV absorber, plasticizer, preservative and also used in drug synthesis, organic synthesis.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. During using it, wear suitable protective clothing. Avoid contact with skin and eyes. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=CC=C(C=C1)OC(=O)C2=CC=CC=C2O
2. InChI: InChI=1S/C13H10O3/c14-12-9-5-4-8-11(12)13(15)16-10-6-2-1-3-7-10/h1-9,14H
3. InChIKey: ZQBAKBUEJOMQEX-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD | intraperitoneal | > 500mg/kg (500mg/kg) | "Summary Tables of Biological Tests," National Research Council Chemical-Biological Coordination Center. Vol. 6, Pg. 149, 1954. | |
| rabbit | LDLo | oral | 3gm/kg (3000mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Revue Medicale de la Suisse Romande. Vol. 15, Pg. 561, 1895. |
| rat | LD50 | oral | 3gm/kg (3000mg/kg) | Food and Cosmetics Toxicology. Vol. 14, Pg. 837, 1976. |
Related Products
- Phenyl (1-piperidinocyclohexyl) ketone
- Phenyl 1-hydroxy-2-naphthoate
- Phenyl 2-bromo-2-propyl ketone
- Phenyl 2-chloroacetate
- Phenyl beta-D-glucopyranoside
- Phenyl bromoacetate
- Phenyl butyrate
- Phenyl carbamate
- PHENYL CARBITOL
- Phenyl chloroformate
- 1185-59-7
- 118564-89-9
- 118-56-9
- 118573-57-2
- 1185768-18-6
- 118-58-1
- 118582-89-1
- 118583-35-0
- 1185897-54-4
- 1186049-67-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View