-
Name
S-Boc-2-mercapto-4,6-dimethylpyrimidine
- EINECS 255-562-1
- CAS No. 41840-28-2
- Density 1.15 g/cm3
- Solubility
- Melting Point 48-51 °C(lit.)
- Formula C11H16N2O2S
- Boiling Point 358.8 °C at 760 mmHg
- Molecular Weight 240.326
- Flash Point 170.8 °C
- Transport Information
- Appearance
- Safety 22-24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms S-tert-Butoxycarbonyl-4,6-dimethyl-2-thiopyrimidine;2-(tert-Butoxycarbonylthio)-4,6-dimethylpyrimidine;
- PSA 77.38000
- LogP 3.12060
S-Boc-2-mercapto-4,6-dimethylpyrimidine Specification
The S-Boc-2-mercapto-4,6-dimethylpyrimidine, with the CAS registry number 41840-28-2 and EINECS registry number 255-562-1, has the systematic name of O-tert-butyl S-(4,6-dimethylpyrimidin-2-yl) carbonothioate. It belongs to the following product categories: Biochemistry; Peptide Synthesis; Protection & Derivatization Reagents (for Synthesis); Protective Reagents (Peptide Synthesis); Synthetic Organic Chemistry. And the molecular formula of the chemical is C11H16N2O2S. What's more, while dealing with this chemical, you should not breathe dust and then try to avoid contacting with skin and eyes.
The characteristics of S-Boc-2-mercapto-4,6-dimethylpyrimidine are as followings: (1)ACD/LogP: 2.95; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.95; (4)ACD/LogD (pH 7.4): 2.95; (5)ACD/BCF (pH 5.5): 102.33; (6)ACD/BCF (pH 7.4): 102.33; (7)ACD/KOC (pH 5.5): 955.8; (8)ACD/KOC (pH 7.4): 955.8; (9)#H bond acceptors: 4; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 77.38 Å2; (13)Index of Refraction: 1.534; (14)Molar Refractivity: 64.62 cm3; (15)Molar Volume: 207.7 cm3; (16)Polarizability: 25.61×10-24cm3; (17)Surface Tension: 47.6 dyne/cm; (18)Density: 1.15 g/cm3; (19)Flash Point: 170.8 °C; (20)Enthalpy of Vaporization: 60.43 kJ/mol; (21)Boiling Point: 358.8 °C at 760 mmHg; (22)Vapour Pressure: 2.48E-05 mmHg at 25°C.
Uses of S-Boc-2-mercapto-4,6-dimethylpyrimidine: It can react with propylamine to produce propyl-carbamic acid tert-butyl ester. This reaction will need reagent triethylamine, and the menstruum dioxane. The reaction time is 6 hours, and the yield is about 93%.
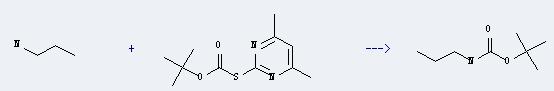
Addtionally, the following datas could be converted into the molecular structure:
(1)SMILES: O=C(OC(C)(C)C)Sc1nc(cc(n1)C)C
(2)InChI: InChI=1/C11H16N2O2S/c1-7-6-8(2)13-9(12-7)16-10(14)15-11(3,4)5/h6H,1-5H3
(3)InChIKey: POTDIELOEHTPJN-UHFFFAOYAW
Related Products
- S-Boc-2-mercapto-4,6-dimethylpyrimidine
- 41840-29-3
- 41840-94-2
- 41841-16-1
- 41847-39-6
- 4184-79-6
- 41851-59-6
- 41859-57-8
- 41859-67-0
- 41860-63-3
- 41860-64-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View