-
Name
Benzeneseleninic acid
- EINECS 230-271-2
- CAS No. 6996-92-5
- Article Data39
- CAS DataBase
- Density 1.9300
- Solubility Slightly soluble in water
- Melting Point 121-124 °C(lit.)
- Formula C6H6O2Se
- Boiling Point 114.4 °C at 760 mmHg
- Molecular Weight 189.072
- Flash Point 23 °C
- Transport Information UN 3283 6.1/PG 2
- Appearance light yellow powder.
- Safety 20/21-28-45-60-61
- Risk Codes 23/25-33-50/53
-
Molecular Structure
-
Hazard Symbols
 T;
T;  N
N
- Synonyms Benzene selenoic acid;Phenylseleninic acid;Phenyl-selensaeure;
- PSA 37.30000
- LogP 0.18480
Seleninobenzoic acid Consensus Reports
Selenium and its compounds are on the Community Right-To-Know List.
Seleninobenzoic acid Standards and Recommendations
OSHA PEL: TWA 0.2 mg(Se)/m3
ACGIH TLV: TWA 0.2 mg(Se)/m3
DFG MAK: 0.1 mg(Se)/m3
Seleninobenzoic acid Specification
The IUPAC name of this chemical is Benzeneselenic acid. With the CAS registry number 6996-92-5 and EINECS registry number 230-271-2, it is also named as Phenylseleninic acid. In addition, the molecular formula is C6H6O2Se and the molecular weight is 189.07. It is a kind of white to beige crystalline powder or needles and belongs to the classes of Pharmaceutical Intermediates; Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts; Classes of Metal Compounds; Oxidation; Se (Selenium) Compounds; Semimetal Compounds; Synthetic Organic Chemistry; Building Blocks; Organic Building Blocks; Selenium Compounds.
Physical properties about this chemical are: (1)#H bond acceptors: 2; (2)#H bond donors: 1; (3)#Freely Rotating Bonds: 1; (4)Polar Surface Area: 26.3 Å2; (5)Flash Point: 23 °C; (6)Enthalpy of Vaporization: 37.33 kJ/mol; (7)Boiling Point: 114.4 °C at 760 mmHg; (8)Vapour Pressure: 15.7 mmHg at 25°C; (9)Exact Mass: 189.953301; (10)MonoIsotopic Mass: 189.953301; (11)Topological Polar Surface Area: 37.3; (12)Heavy Atom Count: 9; (13)Complexity: 108 ; (14)Covalently-Bonded Unit Count: 1.
Preparation of Benzeneselenic acid: it can be prepared by diphenyl-diselane. This reaction will need reagent 30percent H2O2 and solvent dioxane. The reaction time is 10 hours at reaction temperature of 5-10 °C. The yield is about 71%.
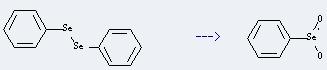
Uses of Benzeneselenic acid: it can react with 2,3-dihydro-indole to get 3-phenylselanyl-1H-indole. This reaction will need solvent tetrahydrofuran. The yield is about 93% at reaction temperature of 20 °C.
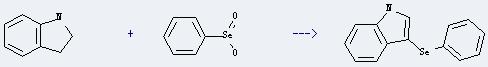
When you are using this chemical, please be cautious about it as the following:
This chemical is toxic by inhalation and if swallowed. And it is very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. In addition, it has danger of cumulative effects. When you are using, do not eat, drink or smoke. If you contact it with your skin, wash it immediately with plenty of ... (to be specified by the manufacturer). In case of accident or if you feel unwell, seek medical advice immediately (show label where possible). This material and/or its container must be disposed of as hazardous waste. What's more, avoid release to the environment. You can refer to special instructions safety data sheet.
You can still convert the following datas into molecular structure:
(1)SMILES: O=[Se](O)c1ccccc1
(2)InChI: InChI=1/C6H6O2Se/c7-9(8)6-4-2-1-3-5-6/h1-5H,(H,7,8)
(3)InChIKey: WIHKGDVGLJJAMC-UHFFFAOYAT
Related Products
- Seleninobenzoic acid
- 69971-79-5
- 699-73-0
- 69974-55-6
- 69975-65-1
- 69975-66-2
- 69975-68-4
- 69975-69-5
- 69975-86-6
- 69978-82-1
- 69980-24-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View