-
Name
STRONTIUM CHLORATE
- EINECS
- CAS No. 7791-10-8
- Density g/cm3
- Solubility g/100g solution H2O: 61.40 (0°C), 63.78 (25°C), 67.08 (95°C); solid phase, Sr(ClO3)2 ·3H2O (0°C), Sr(ClO3)2 (25°C, 95°C) [KRU93]; slightly soluble alcohol [MER06]
- Melting Point 120(分解)oC
- Formula C12O6•Sr
- Boiling Point °Cat760mmHg
- Molecular Weight 327.74
- Flash Point °C
- Transport Information
- Appearance
- Safety A powerful oxidizer. When heated to decomposition it emits toxic fumes of Cl−. See also CHLORATES.
- Risk Codes
-
Molecular Structure
- Hazard Symbols Dangerous explosion risk in contact with organic materials; highly sensitive to shock, heat, and friction; strong oxidizing agent.
- Synonyms EINECS 232-239-3;Chloric acid,strontium salt;strontium dichlorate;STRONTIUM CHLORATE;
- PSA 114.40000
- LogP 0.66620
Strontium chlorate Chemical Properties
IUPAC Name: Strontium dichlorate
Synonyms: Strontium chlorate ; Strontium chlorate [UN1506] [Oxidizer]
CAS NO: 7791-10-8
Molecular Formula of Strontium chlorate (CAS NO.7791-10-8) : Cl2O6Sr
Molecular Weight of Strontium chlorate (CAS NO.7791-10-8) : 254.52
Molecular Structure of Strontium chlorate (CAS NO.7791-10-8) :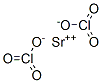
EINECS: 232-239-3
Appearance:A moist solid or semi-solid slurry of white crystals.
Appearance: colorless or white crystalline powder.
Solubility: soluble in water, slightly soluble in alcohol. Strong oxidant
Strontium chlorate Production
Raw materials: Strontium chloride
Adding Sodium chlorate solution into the reactor,adding Anhydrous strontium chloride solution, heated for metathesis reaction to generate Strontium acid and Sodium chloride, filtered to remove Sodium chloride, the filtrate suitable, evaporation, cooling crystallization, centrifugal separation,obtaining Strontium acid . Its
2NaC1O3 + SrC12 → Sr (C1O3) 2 +2 NaCl
Strontium chlorate Consensus Reports
Reported in EPA TSCA Inventory.
Strontium chlorate Safety Profile
A powerful oxidizer. When heated to decomposition it emits toxic fumes of Cl−. See also CHLORATES.
RIDADR 1506
HazardClass 5.1
PackingGroup II
Strontium chlorate Standards and Recommendations
DOT Classification: 5.1; Label: Oxidizer
Strontium chlorate Specification
1.Reactivity Profile: Strontium chlorate is a strong oxidizing agent. Forms a flammable mixture with combustible materials (can be ignited by friction). Such mixtures may be explosive Contact with concentrated sulfuric acid can cause fires or explosions. When mixed with ammonium salts, spontaneous decomposition and ignition may result. Liberates explosive chlorine dioxide gas in the presence of strong acid; heating with a dibasic organic acid in the presence of moisture liberates chlorine dioxide and carbon dioxide Mixtures with ammonium salts, powdered metals, silicon, sulfur, or sulfides are readily ignited and potentially explosive
2.Health Hazard :TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Fire may produce irritating and/or toxic gases. Toxic fumes or dust may accumulate in confined areas (basement, tanks, hopper/tank cars, etc.). Runoff from fire control or dilution water may cause pollution.
3.Fire Hazard :May explode from friction, heat or contamination. These substances will accelerate burning when involved in a fire. May ignite combustibles (wood, paper, oil, clothing, etc.). Some will react explosively with hydrocarbons (fuels). Containers may explode when heated. Runoff may create fire or explosion hazard.
Related Products
- STRONTIUM ACETATE
- Strontium acetylide
- Strontium adipate
- Strontium arsenite
- strontium bis(dihydrogen phosphate)
- Strontium bis(mercaptoacetate)
- Strontium bromide
- Strontium bromide(SrBr<sub>2</sub>), hexahydrate (9CI)
- Strontium carbonate
- Strontium chlorate
- 7791-11-9
- 7791-12-0
- 7791-13-1
- 7791-16-4
- 7791-18-6
- 7791-20-0
- 7791-23-3
- 7791-25-5
- 7791-28-8
- 7791-49-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View