-
Name
Tebufenozide
- EINECS 412-850-3
- CAS No. 112410-23-8
- Article Data8
- CAS DataBase
- Density 1.074 g/cm3
- Solubility 0.83 mg l-1 (20 °C)
- Melting Point 191oC
- Formula C22H28N2O2
- Boiling Point
- Molecular Weight 352.477
- Flash Point
- Transport Information
- Appearance
- Safety 61
- Risk Codes 51/53
-
Molecular Structure
-
Hazard Symbols
 N
N
- Synonyms Mimic;Mimic 240LV;Mimic 700WP;RH 5992;Romdan;
- PSA 49.41000
- LogP 4.84240
Synthetic route
-
-
6613-44-1
3,5-dimethylbenzoyl chloride

-
-
139303-74-5
1-(4-ethylbenzoyl)-2-t-butyl hydrazine

-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| With sodium hydroxide In diethyl ether for 48h; Ambient temperature; |
-
-
16331-45-6
p-ethylbenzoyl chloride

-
-
162752-59-2
N-(3,5-Dimethylbenzoyl)-N-tert-Butyl-Hydrazine

-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dichloromethane; water at 20℃; |
-
-
499-06-9
3,5-dimethylbenzoic acid

-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 84.2 percent / SOCl2 / 5 h / 80 °C 2: 1N aq. NaOH / diethyl ether / 48 h / Ambient temperature View Scheme |
-
-
619-64-7
p-ethylbenzoic acid

-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: SOCl2 / 5 h / 80 °C 2: aq. NaOH / dioxane / 48 h / Ambient temperature 3: 1N aq. NaOH / diethyl ether / 48 h / Ambient temperature View Scheme |
-
-
16331-45-6
p-ethylbenzoyl chloride

-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. NaOH / dioxane / 48 h / Ambient temperature 2: 1N aq. NaOH / diethyl ether / 48 h / Ambient temperature View Scheme |
-
-
16331-45-6
p-ethylbenzoyl chloride

-
-
7400-27-3
tertbutylhydrazine hydrochloride

-
-
6613-44-1
3,5-dimethylbenzoyl chloride

-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| With sodium hydroxide In toluene |

-
-
75-44-5
phosgene

-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With potassium tert-butylate In tetrahydrofuran at 20℃; for 1h; Stage #2: phosgene In tetrahydrofuran; ethyl acetate at -78℃; for 0.5h; | 95% |
-
-
463-71-8
thiophosgene

-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With potassium tert-butylate In tetrahydrofuran at 20℃; Stage #2: thiophosgene In tetrahydrofuran; ethyl acetate at -78℃; | 80% |
-
-
105-36-2
ethyl bromoacetate

-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With sodium hydride In N,N-dimethyl-formamide at 20℃; for 2h; Stage #2: ethyl bromoacetate In N,N-dimethyl-formamide at 35 - 40℃; for 10h; | 62% |
-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| With pyridine; oxalyl dichloride In 1,2-dichloro-ethane at 20℃; for 6h; | 60.8% |
| With pyridine; oxalyl dichloride In dichloromethane at 20℃; |
-
-
1051431-26-5
chlorosulfenyl[O-(1-methylthioethylimino)-N-methylcarbamate]

-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With sodium hydride In xylene for 2h; Heating; Stage #2: chlorosulfenyl[O-(1-methylthioethylimino)-N-methylcarbamate] In xylene at 20℃; for 6h; Further stages.; | 52% |
-
-
96-32-2
bromoacetic acid methyl ester

-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With sodium hydride In N,N-dimethyl-formamide at 20℃; for 2h; Stage #2: bromoacetic acid methyl ester In N,N-dimethyl-formamide at 35 - 40℃; for 10h; | 50% |
-
-
79-37-8
oxalyl dichloride

-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-ethane for 8h; Heating; | A 45.3% B 32% |
| In 1,2-dichloro-ethane Heating; |


-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With sodium hydride In tetrahydrofuran at 20℃; for 0.5h; Stage #2: chlorooxalyl-methyl-carbamic acid m-tolyl ester In tetrahydrofuran at 20℃; for 4h; | 42.8% |

-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With sodium hydride In tetrahydrofuran at 20℃; for 0.5h; Stage #2: chlorooxalyl-methyl-carbamic acid 2-isopropyl-phenyl ester In tetrahydrofuran at 20℃; for 4h; | 40.5% |
-
-
51719-70-1
phenyl oxalyl chloride

-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With sodium hydride In tetrahydrofuran at 20℃; for 1h; Stage #2: phenyl oxalyl chloride In tetrahydrofuran at 20℃; for 5h; |
-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With sodium hydride In tetrahydrofuran at 20℃; for 1h; Stage #2: 4-chlorooxalyloxy-benzoic acid methyl ester In tetrahydrofuran at 20℃; for 5h; |
-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With sodium hydride In tetrahydrofuran at 20℃; for 1h; Stage #2: 2-chlorooxalyloxy-benzoic acid methyl ester In tetrahydrofuran at 20℃; for 5h; |
-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With sodium hydride In tetrahydrofuran at 20℃; for 1h; Stage #2: 3-chlorooxalyloxy-benzoic acid methyl ester In tetrahydrofuran at 20℃; for 5h; |
-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With sodium hydride In tetrahydrofuran at 20℃; for 1h; Stage #2: 4-chlorooxalyloxy-benzoic acid isopropyl ester In tetrahydrofuran at 20℃; for 5h; |
-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With sodium hydride In tetrahydrofuran at 20℃; for 1h; Stage #2: 2-chlorooxalyloxy-benzoic acid isopropyl ester In tetrahydrofuran at 20℃; for 5h; |
-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With sodium hydride In tetrahydrofuran at 20℃; for 1h; Stage #2: 3-chlorooxalyloxy-benzoic acid isopropyl ester In tetrahydrofuran at 20℃; for 5h; |
-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With sodium hydride In tetrahydrofuran at 20℃; for 1h; Stage #2: 4-chlorooxalyloxy-benzoic acid butyl ester In tetrahydrofuran at 20℃; for 5h; |
-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With sodium hydride In tetrahydrofuran at 20℃; for 1h; Stage #2: 2-chlorooxalyloxy-benzoic acid butyl ester In tetrahydrofuran at 20℃; for 5h; |
-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With sodium hydride In tetrahydrofuran at 20℃; for 1h; Stage #2: 3-chlorooxalyloxy-benzoic acid butyl ester In tetrahydrofuran at 20℃; for 5h; |
-
-
112410-23-8
tebufenozide

-
-
869878-47-7
4-chlorooxalyloxy-benzoic acid benzyl ester

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With sodium hydride In tetrahydrofuran at 20℃; for 1h; Stage #2: 4-chlorooxalyloxy-benzoic acid benzyl ester In tetrahydrofuran at 20℃; for 5h; |
-
-
112410-23-8
tebufenozide

-
-
869879-62-9
2-chlorooxalyloxy-benzoic acid benzyl ester

-
-
784186-29-4
2-{[N'-tert-butyl-N'-(3,5-dimethyl-benzoyl)-N-(4-ethyl-benzoyl)-hydrazino]-oxo-acetoxy}-benzoic acid benzyl ester

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With sodium hydride In tetrahydrofuran at 20℃; for 1h; Stage #2: 2-chlorooxalyloxy-benzoic acid benzyl ester In tetrahydrofuran at 20℃; for 5h; |
-
-
112410-23-8
tebufenozide

-
-
869881-36-7
3-chlorooxalyloxy-benzoic acid benzyl ester

-
-
784186-37-4
3-{[N'-tert-butyl-N'-(3,5-dimethyl-benzoyl)-N-(4-ethyl-benzoyl)-hydrazino]-oxo-acetoxy}-benzoic acid benzyl ester

| Conditions | Yield |
|---|---|
| Stage #1: tebufenozide With sodium hydride In tetrahydrofuran at 20℃; for 1h; Stage #2: 3-chlorooxalyloxy-benzoic acid benzyl ester In tetrahydrofuran at 20℃; for 5h; |
-
-
112410-23-8
tebufenozide

-
-
1037075-79-8
C22H27ClN2O2S

| Conditions | Yield |
|---|---|
| With pyridine; sulfur dichloride In dichloromethane at 20℃; for 4h; |
-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 45.3 percent / 1,2-dichloro-ethane / 8 h / Heating 2: 92.7 percent / 2 h / Heating View Scheme |
-
-
112410-23-8
tebufenozide

-
-
84270-05-3
4-ethyl-N-phenyl benzamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: tBuOK / tetrahydrofuran / 1 h / 20 °C 1.2: 95 percent / tetrahydrofuran; ethyl acetate / 0.5 h / -78 °C 2.1: 300 mg / tetrahydrofuran / 4 h View Scheme |
-
-
112410-23-8
tebufenozide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: tBuOK / tetrahydrofuran / 1 h / 20 °C 1.2: 95 percent / tetrahydrofuran; ethyl acetate / 0.5 h / -78 °C 2.1: 250 mg / tetrahydrofuran / 4 h View Scheme |
Tebufenozide Specification
The Tebufenozide technical is an organic compound with the formula C22H28N2O2. The IUPAC name of this chemical is N-tert-butyl-N'-(4-ethylbenzoyl)-3,5-dimethylbenzohydrazide. With the CAS registry number 112410-23-8, it is also named as 1-(1,1-Dimethylethyl)-2-(4-ethylbenzoyl)hydrazide 3,5-dimethylbenzoate. The product's categories are Growth regulatorsPesticides & Metabolites; Alpha sort; Insecticides; Pesticides; Q-ZAlphabetic; TA - TE. Besides, it should be stored in a closed cool and dry place. It have the stomach poison function, it is a ecdysone agonist and can induce lepidopterous larvae molt in advance before its stage of molting. It is used to control most of the lepidopteron insects such as beet armyworm, cabbage armyworm, tortricid, pine cater pillar etc.
Physical properties about Tebufenozide technical are: (1)ACD/LogP: 4.24; (2)ACD/LogD (pH 5.5): 4.24; (3)ACD/LogD (pH 7.4): 4.24; (4)ACD/BCF (pH 5.5): 985.37; (5)ACD/BCF (pH 7.4): 985; (6)ACD/KOC (pH 5.5): 4835.24; (7)ACD/KOC (pH 7.4): 4833.41; (8)#H bond acceptors: 4; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 5; (11)Polar Surface Area: 40.62 Å2; (12)Index of Refraction: 1.561; (13)Molar Refractivity: 106.25 cm3; (14)Molar Volume: 327.8 cm3; (15)Polarizability: 42.12×10-24cm3; (16)Surface Tension: 41.6 dyne/cm; (17)Density: 1.074 g/cm3.
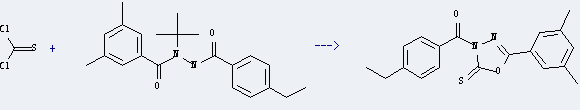
Preparation: this chemical can be prepared by Oxadiazole ring. Reaction equation is as follows:
.gif)
Uses of Tebufenozide technical: it can be used to produce [5-(3,5-dimethyl-phenyl)-2-thioxo-[1,3,4]oxadiazol-3-yl]-(4-ethyl-phenyl)-methanone at temperature of 200 °C. It will need reagent tBuOK and solvent tetrahydrofuran, tetrahydrofuran, ethyl acetate. The yield is about 80%.
When you are using this chemical, please be cautious about it as the following:
It is toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. When you are using it, avoid release to the environment. Refer to special instructions/safety data sheet.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(c1cc(cc(c1)C)C)N(NC(=O)c2ccc(cc2)CC)C(C)(C)C
(2)InChI: InChI=1/C22H28N2O2/c1-7-17-8-10-18(11-9-17)20(25)23-24(22(4,5)6)21(26)19-13-15(2)12-16(3)14-19/h8-14H,7H2,1-6H3,(H,23,25)
(3)InChIKey: QYPNKSZPJQQLRK-UHFFFAOYAS
(4)Std. InChI: InChI=1S/C22H28N2O2/c1-7-17-8-10-18(11-9-17)20(25)23-24(22(4,5)6)21(26)19-13-15(2)12-16(3)14-19/h8-14H,7H2,1-6H3,(H,23,25)
(5)Std. InChIKey: QYPNKSZPJQQLRK-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View