-
Name
4-BROMOPHENYLTHIOUREA
- EINECS -0
- CAS No. 2646-30-2
- Article Data50
- CAS DataBase
- Density 1.728 g/cm3
- Solubility
- Melting Point 183-184 °C
- Formula C7H7BrN2S
- Boiling Point 317 °C at 760 mmHg
- Molecular Weight 231.116
- Flash Point 145.5 °C
- Transport Information
- Appearance
- Safety 22-36/37-45
- Risk Codes 25
-
Molecular Structure
- Hazard Symbols
- Synonyms 4-Bromophenylthiourea;N-(4-Bromophenyl)thiourea;N-p-Bromophenylthiourea;Urea, 1-(p-bromophenyl)-2-thio- (6CI,7CI,8CI);1-(4-Bromophenyl)thiourea;Thiourea,(4-bromophenyl)- (9CI);NSC 3404;NSC 72167;p-Bromophenylthiourea;
- PSA 70.14000
- LogP 2.87790
Synthetic route
-
-
333-20-0
potassium thioacyanate

-
-
624-19-1
4-bromoaniline hydrochloride

-
-
2646-30-2
1-(4-bromophenyl)thiourea

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Addition; Heating; | 96% |
-
-
19249-89-9
1-benzoyl-3-(4-bromophenyl)thiourea

-
-
2646-30-2
1-(4-bromophenyl)thiourea

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 85℃; for 0.2h; | 87.5% |
| With sodium hydroxide; water In ethanol for 1h; Heating / reflux; | 70% |
| With sodium hydroxide; water for 0.5h; Heating / reflux; | 60% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water for 0.5h; | 76.5% |
| Stage #1: ammonium thiocyanate; 4-bromo-aniline With hydrogenchloride In water for 1h; Heating; Stage #2: Heating; | 28.55% |
-
-
4137-02-4
3-(4-Bromphenyl)-4-oxo-2-thioxo-tetrahydro-1,3-thiazin

-
-
2646-30-2
1-(4-bromophenyl)thiourea

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In ethanol for 1h; Heating; | 74% |

| Conditions | Yield |
|---|---|
| With ammonia | |
| With ammonia In ethanol Yield given; | |
| With ammonia; water In tetrahydrofuran at 20℃; for 0.166667h; |
-
-
19249-89-9
1-benzoyl-3-(4-bromophenyl)thiourea

-
-
124-41-4
sodium methylate

-
A
-
93-58-3
benzoic acid methyl ester

-
B
-
2646-30-2
1-(4-bromophenyl)thiourea

| Conditions | Yield |
|---|---|
| In methanol at 25℃; Rate constant; 4-bromophenolate buffer; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: acetone 2: NaOH View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: acetone / 0.25 h / Heating 1.2: acetone / 0.5 h / Heating 2.1: aq. NaOH View Scheme |
-
-
1147550-11-5
ammonium thiocyanate

-
-
624-19-1
4-bromoaniline hydrochloride

-
-
2646-30-2
1-(4-bromophenyl)thiourea

| Conditions | Yield |
|---|---|
| Stage #1: 4-bromoaniline hydrochloride With hydrogenchloride In water for 0.5h; Reflux; Stage #2: ammonium thiocyanate In water at 20℃; for 4h; Reflux; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: calcium carbonate / water; dichloromethane / 19.5 h / 0 - 25 °C 2: ammonia / tetrahydrofuran / 25 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: acetone / 0.17 h / 20 °C 1.2: 20 °C 2.1: bis(trichloromethyl) carbonate / chloroform / 1 h 3.1: ammonium hydroxide / dichloromethane / 3 h / 0 °C View Scheme | |
| Multi-step reaction with 2 steps 1: acetonitrile / 0.17 h / Milling; Green chemistry 2: ammonium chloride; sodium carbonate / neat (no solvent) / 1 h / Milling; Green chemistry View Scheme |
-
-
1414781-39-7
C7H6BrNS2*C6H12N2

-
-
2646-30-2
1-(4-bromophenyl)thiourea

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: bis(trichloromethyl) carbonate / chloroform / 1 h 2: ammonium hydroxide / dichloromethane / 3 h / 0 °C View Scheme |

-
-
2646-30-2
1-(4-bromophenyl)thiourea

| Conditions | Yield |
|---|---|
| With sodium carbonate; ammonium chloride In neat (no solvent) for 1h; Reagent/catalyst; Solvent; Milling; Green chemistry; |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In Isopropyl acetate for 16h; Reflux; |
-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
774544-68-2
N-(4-bromophenyl)thiazol-2-amine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water for 2h; Reflux; | 100% |
| With hydrogenchloride In water for 2h; Hantzsch Thiazole Synthesis; Reflux; |
-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
70-11-1
α-bromoacetophenone

-
-
108237-91-8
4-bromo-phenyl-(4-phenyl-thiazol-2-yl)-amine

| Conditions | Yield |
|---|---|
| In ethanol at 80℃; under 1500.15 Torr; | 98% |
| With Nafion-H In water; ethylene glycol at 50℃; for 0.166667h; | 96% |
| In glycerol at 90℃; for 2h; | 94% |
| With triethylamine In ethanol Reflux; | 82% |
| at 80℃; under 1500.15 Torr; Flow reactor; Green chemistry; |

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-bromophenyl)thiourea; ethyl acetoacetate; meta-hydroxybenzaldehyde In N,N-dimethyl-formamide for 1h; Biginelli Pyrimidone Synthesis; Sonication; Stage #2: With chloro-trimethyl-silane In N,N-dimethyl-formamide at 20℃; for 72h; Biginelli Pyrimidone Synthesis; | 98% |

-
-
13987-61-6
N-methylene-tert-butylamine

-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
140628-23-5
1-(4-Bromo-phenyl)-5-tert-butyl-[1,3,5]triazinane-2-thione

| Conditions | Yield |
|---|---|
| for 4h; Heating; | 96% |
-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
61397-54-4
2-bromo-1-(2-chloro-4-fluorophenyl)ethan-1-one

-
-
1239982-56-9
2-(4-bromophenylamino)-4-(2-chloro-4-fluorophenyl)thiazole

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-bromophenyl)thiourea; 2-bromo-1-(2-chloro-4-fluorophenyl)ethan-1-one In ethanol at 30 - 35℃; for 1.25h; Hantzsch reaction; Sonication; Stage #2: With ammonium hydroxide In ethanol | 96% |
-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
63529-30-6
2-bromo-3′-chloro-4′-fluoroacetophenone

-
-
1239982-55-8
2-(4-bromophenylamino)-4-(3-chloro-4-fluorophenyl)thiazole

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-bromophenyl)thiourea; 2-bromo-3′-chloro-4′-fluoroacetophenone In ethanol at 30 - 35℃; for 1.25h; Hantzsch reaction; Sonication; Stage #2: With ammonium hydroxide In ethanol | 96% |
-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
74-88-4
methyl iodide

-
-
73318-38-4
N-(p-Bromphenyl)-S-methyl-isothioharnstoff

| Conditions | Yield |
|---|---|
| In acetone at 20℃; for 23.6667h; | 93% |
-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
403-29-2
2-bromo-4'-fluoroacetophenone

-
-
61383-57-1
2-(4-bromophenylamino)-4-(4-fluorophenyl)thiazole

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-bromophenyl)thiourea; 2-bromo-4'-fluoroacetophenone In ethanol at 30 - 35℃; for 2.25h; Hantzsch reaction; Sonication; Stage #2: With ammonium hydroxide In ethanol | 92.4% |

| Conditions | Yield |
|---|---|
| at 100℃; for 0.25h; Sealed tube; Microwave irradiation; | 92% |
| Sealed tube; Microwave irradiation; | 92% |

-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
99-73-0
para-bromophenacyl bromide

-
-
107943-12-4
2-(4-bromophenylamino)-4-(4-bromophenyl)thiazole

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-bromophenyl)thiourea; para-bromophenacyl bromide In ethanol at 30 - 35℃; for 1.5h; Hantzsch reaction; Sonication; Stage #2: With ammonium hydroxide In ethanol | 91.5% |
-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
26551-48-4
Methyl 3-bromo-4-keto-4-phenylbutanoate

-
-
729580-96-5
C18H15BrN2O2S

| Conditions | Yield |
|---|---|
| With PEG-400 for 0.0166667h; microwave irradiation; | 90% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In PEG 400 at 80℃; for 2h; | 90% |
-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
60592-84-9
N-(4-bromophenyl)cyanamide

| Conditions | Yield |
|---|---|
| With copper(l) iodide; sodium hydroxide In dimethyl sulfoxide at 90℃; for 3h; | 89% |
| With sodium borate; sodium hydroxide | |
| With potassium hydroxide; lead acetate |
-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
35158-43-1
methyl 3-bromo-(4-chlorobenzoyl)propionate

-
-
264227-26-1
C18H14BrClN2O2S

| Conditions | Yield |
|---|---|
| With PEG-400 for 0.0166667h; microwave irradiation; | 89% |
-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
63529-31-7
2-bromo-1-(4-fluoro-3-methyl-phenyl)-ethanone

-
-
1239982-53-6
2-(4-bromophenylamino)-4-(4-fluoro-3-methylphenyl)thiazole

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-bromophenyl)thiourea; 2-bromo-1-(4-fluoro-3-methyl-phenyl)-ethanone In ethanol at 30 - 35℃; for 2.5h; Hantzsch reaction; Sonication; Stage #2: With ammonium hydroxide In ethanol | 89% |
-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
123771-08-4
3-(2-Bromoacetyl)tropolone

-
-
123771-15-3
3-<2-(4-bromoanilino)-4-thiazolyl>tropolone

| Conditions | Yield |
|---|---|
| In ethanol for 0.5h; Heating; | 87% |
-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
350-27-6
2-bromo-1-(3-fluoro-4-methoxyphenyl)ethanone

-
-
78864-18-3
(4-bromo-phenyl)-[5-(3-fluoro-4-methoxy-phenyl)-thiazol-2-yl]-amine

| Conditions | Yield |
|---|---|
| In ethanol for 8h; Heating; | 86% |
| In ethanol |
-
-
352517-78-3
1-(α-tosyloxy)acetonaphthone

-
-
2646-30-2
1-(4-bromophenyl)thiourea

| Conditions | Yield |
|---|---|
| With potassium carbonate In neat (no solvent) at 50 - 60℃; Green chemistry; | 86% |

| Conditions | Yield |
|---|---|
| With aluminum oxide for 0.116667h; microwave irradiation; | 85% |
-
-
70-23-5
ethyl Bromopyruvate

-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
165682-91-7
ethyl 2-((4-bromophenyl)amino)thiazole-4-carboxylate

| Conditions | Yield |
|---|---|
| With sodium carbonate In neat (no solvent) Milling; | 85% |
| In ethanol Reflux; | 80% |
| In ethanol for 3h; Reflux; |
-
-
115035-43-3
3-bromo-1-benzofuran-2(3H)-one

-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
1628629-77-5
N-(4-bromophenyl)-S-(2-oxo-2,3-dihydro-1-benzofuran-3-yl)isothiuronium bromide

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; | 85% |
-
-
4209-02-3
1-(4-bromophenyl)-2-chloroethan-1-one

-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
107943-12-4
2-(4-bromophenylamino)-4-(4-bromophenyl)thiazole

| Conditions | Yield |
|---|---|
| With potassium carbonate at 20℃; for 8h; Milling; | 84% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-bromophenyl)thiourea; ethyl acetoacetate; benzaldehyde In N,N-dimethyl-formamide for 1h; Biginelli Pyrimidone Synthesis; Sonication; Stage #2: With chloro-trimethyl-silane In N,N-dimethyl-formamide at 20℃; for 72h; Biginelli Pyrimidone Synthesis; | 83% |

-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
98-86-2
acetophenone

-
-
108237-91-8
4-bromo-phenyl-(4-phenyl-thiazol-2-yl)-amine

| Conditions | Yield |
|---|---|
| With carbon tetrabromide; triethylamine In acetonitrile at 20℃; for 4h; | 82% |
| Stage #1: acetophenone With 1,1'-(ethane-1,2-diyl)dipyridinium bistribromide In acetonitrile for 0.75h; Stage #2: 1-(4-bromophenyl)thiourea In water; acetonitrile at 20℃; for 1h; |

| Conditions | Yield |
|---|---|
| With water; potassium carbonate In PEG 400 at 80℃; for 2h; | 82% |
-
-
53336-42-8
3-bromo-2,3-dihydro-thiophene-1,1-dioxide

-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
123414-44-8
(3aR,6aR)-3-(4-Bromo-phenyl)-5,5-dioxo-hexahydro-5λ6-thieno[3,4-d]thiazol-2-ylideneamine; hydrobromide

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 80℃; for 3h; | 81% |
-
-
1116559-13-7
2-bromo-1-(2-methylimidazo[1,2-a]pyrimidin-3-yl)ethanone monohydrobromide

-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
1116559-17-1
2-(4-bromophenylamino)-4-(2-methyl-imidazo[1,2-a]pyrimidin-3-yl)thiazole monohydrobromide

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 26h; Heating / reflux; | 81% |
-
-
2646-30-2
1-(4-bromophenyl)thiourea

-
-
357915-19-6
α-bromo-(2,4-dichloro-5-fluoro)acetophenone

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Heating; | 78% |
Thiourea,N-(4-bromophenyl)- Specification
The Thiourea,N-(4-bromophenyl)-, with the CAS registry number 2646-30-2, has the systematic name of 1-(4-bromophenyl)thiourea. It belongs to the product category of Heterocycles. The molecular formula of the chemical is C7H7BrN2S, and its molecular weight is 231.1129.
The characteristics of Thiourea,N-(4-bromophenyl)- are as followings: (1)ACD/LogP: 1.90; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.9; (4)ACD/LogD (pH 7.4): 1.9; (5)ACD/BCF (pH 5.5): 16.27; (6)ACD/BCF (pH 7.4): 16.27; (7)ACD/KOC (pH 5.5): 256.34; (8)ACD/KOC (pH 7.4): 256.33; (9)#H bond acceptors: 2; (10)#H bond donors: 3; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 38.57 Å2; (13)Index of Refraction: 1.748; (14)Molar Refractivity: 54.37 cm3; (15)Molar Volume: 133.7 cm3; (16)Polarizability: 21.55×10-24cm3; (17)Surface Tension: 73.9 dyne/cm; (18)Density: 1.728 g/cm3; (19)Flash Point: 145.5 °C; (20)Enthalpy of Vaporization: 55.84 kJ/mol; (21)Boiling Point: 317 °C at 760 mmHg; (22)Vapour Pressure: 0.000395 mmHg at 25°C.
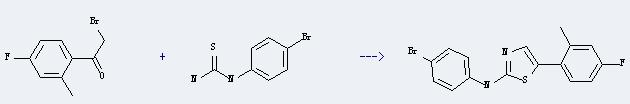
Uses of Thiourea,N-(4-bromophenyl)-: It can react with 2-bromo-1-(4-fluoro-2-methyl-phenyl)-ethanone to produce (4-bromo-phenyl)-[5-(4-fluoro-2-methyl-phenyl)-thiazol-2-yl]-amine. This reaction will need menstruum ethanol. The reaction time is 8 hours with heating, and the yield is about 60%.
You should be cautious while dealing with this chemical. It is toxic if swallowed. Therefore, you had better take the following instructions: Do not breathe dust; Wear suitable protective clothing and gloves, and if in case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
Addtionally, the following datas could be converted into the molecular structure:
(1)SMILES: Brc1ccc(NC(=S)N)cc1
(2)InChI: InChI=1/C7H7BrN2S/c8-5-1-3-6(4-2-5)10-7(9)11/h1-4H,(H3,9,10,11)
(3)InChIKey: MRVQULNOKCOGHC-UHFFFAOYAE
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LDLo | oral | 50mg/kg (50mg/kg) | National Academy of Sciences, National Research Council, Chemical-Biological Coordination Center, Review. Vol. 5, Pg. 46, 1953 |
Related Products
- Thiourea
- THIOUREA DIOXIDE
- Thiourea dioxide
- Thiourea, compd. with (3-chloropropyl)silanetriol (1:1)
- Thiourea, hydroxy-
- Thiourea, N-(1,2,3,4-tetrahydro-2,4-dioxo-5-pyrimidinyl)-
- Thiourea, N-(2,4,6-trichlorophenyl)-
- Thiourea, N-(2-aminophenyl)-N-phenyl-
- Thiourea, N-(3,6-dihydroxy-3-oxospiro(isobenzofuran-1(3H),9-(9H)xanthen)-5-yl)-N-(9-(2-octylcyclopropyl)nonyl)-
- Thiourea, N-(3-chloro-2-methylphenyl)-N'-phenyl-
- 2646-31-3
- 26464-05-1
- 26465-81-6
- 2646-71-1
- 26468-79-1
- 26468-86-0
- 2646-90-4
- 2646-91-5
- 2647-14-5
- 26471-62-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View