-
Name
Tigogenin
- EINECS 201-041-9
- CAS No. 77-60-1
- Article Data71
- CAS DataBase
- Density 1.11 g/cm3
- Solubility
- Melting Point 202-204 °C
- Formula C27H44O3
- Boiling Point 516.6 °C at 760 mmHg
- Molecular Weight 416.645
- Flash Point 266.2 °C
- Transport Information
- Appearance White, micro-cryst. powder
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 5a-Spirostan-3b-ol (7CI);5a-Spirostan-3b-ol, (25R)- (8CI);(25R)-5a-Spirostan-3b-ol;
- PSA 38.69000
- LogP 5.79380
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen; palladium dihydroxide In ethanol; dichloromethane | 99% |
| With hydrogen; acetic acid; palladium on activated charcoal In ethanol; dichloromethane at 20℃; under 760 Torr; for 12h; | 93% |
| With 5% Pd/C; hydrogen In ethyl acetate at 20℃; under 3750.38 Torr; | |
| With 5%-palladium/activated carbon; hydrogen In methanol at 20℃; for 3h; |

| Conditions | Yield |
|---|---|
| Stage #1: hecogenin acetate With hydrazine hydrate In 2-ethoxy-ethanol at 136℃; Inert atmosphere; Stage #2: With potassium hydroxide In 2-ethoxy-ethanol at 136℃; for 3h; Inert atmosphere; | 98% |
-
-
61046-18-2
(25R)-5α-Spirostan-2β,3α-diol

-
-
77-60-1
tigogenin

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen; acetic acid under 1500.15 Torr; for 24h; | 98% |
-
-
690275-05-9
C39H72N2O3Si2

-
-
77-60-1
tigogenin

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; tert-butyl alcohol In dimethyl sulfoxide at 100℃; for 24h; | 95% |

-
-
77-60-1
tigogenin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol for 3h; Heating; | 57.9% |
-
-
67-56-1
methanol

-
A
-
77-60-1
tigogenin

-
B
-
97-30-3, 617-04-9, 709-50-2, 1824-94-8, 2152-78-5, 3396-99-4, 6819-48-3, 18469-06-2, 20550-04-3, 22277-65-2, 29411-57-2, 35437-40-2, 35437-43-5, 36191-11-4, 36191-12-5, 39598-79-3, 39598-80-6, 39598-89-5, 51023-63-3, 51223-62-2, 51224-38-5, 51224-39-6, 51224-40-9, 51224-41-0, 51819-81-9, 51819-82-0, 51819-83-1, 56688-81-4, 62279-53-2, 64281-79-4, 64281-80-7, 64912-19-2, 66101-73-3, 78184-90-4, 84368-39-8, 84368-40-1, 84368-41-2, 92142-37-5, 93302-26-2, 103421-28-9, 115794-08-6, 3149-68-6
methyl D-glucopyranoside

-
C
-
91-09-8, 612-05-5, 1825-00-9, 2500-78-9, 3867-83-2, 3945-28-6, 5328-63-2, 6206-67-3, 6207-01-8, 6207-03-0, 7404-24-2, 17289-61-1, 18449-76-8, 33509-64-7, 36793-06-3, 41897-31-8, 41897-32-9, 41897-33-0, 41897-34-1, 41897-37-4, 41897-38-5, 41897-39-6, 53448-52-5, 65137-86-2, 89615-04-3, 89615-05-4, 131233-91-5, 14703-09-4
methyl xyloside

-
D
-
1128-40-1, 1198-82-9, 2592-55-4, 5155-43-1, 6340-52-9, 10503-91-0, 14009-07-5, 14687-15-1, 14917-55-6, 15814-59-2, 24332-98-7, 34299-65-5, 42214-00-6, 42214-11-9, 57783-34-3, 57783-36-5, 57783-38-7, 57783-40-1, 57783-42-3, 58525-46-5, 58525-47-6, 58525-48-7, 62182-01-8, 62182-02-9, 65310-00-1, 65941-62-0, 68069-80-7, 71116-57-9, 72274-54-5, 72274-55-6, 73036-22-3, 83198-64-5, 85505-15-3, 102517-74-8, 109718-80-1, 109718-81-2, 125280-09-3, 129172-95-8, 63864-94-8
methyl L-rhamnopyranoside

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 2.5h; Product distribution; Heating; | A 51.5% B n/a C n/a D n/a |


| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether for 3h; Ambient temperature; | A 17% B 8% |
-
-
64-17-5
ethanol

-
-
108990-28-9
(25R)-22-ethoxy-5α,22αH-furostan-3β,26-diol

-
-
64-19-7
acetic acid

-
-
77-60-1
tigogenin

-
-
64-17-5
ethanol

-
-
118633-83-3
(25R)-5α-furost-22ξ-ene-3β,26-diol

-
-
64-19-7
acetic acid

-
-
77-60-1
tigogenin

-
-
118633-83-3
(25R)-5α-furost-22ξ-ene-3β,26-diol

-
-
77-60-1
tigogenin

| Conditions | Yield |
|---|---|
| at 150℃; |
-
-
122386-94-1
(25R)-3β.26-dihydroxy-5α-cholestanedione-(16.22)

-
-
555-31-7
aluminum isopropoxide

-
-
67-63-0
isopropyl alcohol

-
-
77-60-1
tigogenin

-
-
67-56-1
methanol

-
A
-
3149-37-9, 3149-38-0, 23009-68-9, 23262-64-8, 23397-56-0, 27552-00-7, 31085-65-1, 51385-14-9
methyl 2,3,6-tri-O-methyl α-D-galactopyranoside

-
B
-
35939-71-0
methyl 2,4,6-tri-O-methyl-α-D-glucopyranoside

-
C
-
77-60-1
tigogenin

-
D
-
2296-43-7, 2876-84-8, 2876-85-9, 2876-86-0, 2876-87-1, 2876-90-6, 7381-06-8, 14187-58-7, 14550-94-8, 20188-52-7, 50773-45-0, 72984-55-5, 90760-77-3
methyl 2,3,4-tri-O-methyl α,β-D-xylopyranoside

-
E
-
82262-93-9
methyl 4,6-di-O-methyl α,β-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With hydrogenchloride Product distribution; Heating; |


-
-
77-60-1
tigogenin

| Conditions | Yield |
|---|---|
| With sulfuric acid In water at 110℃; for 10h; |

-
-
77-60-1
tigogenin

| Conditions | Yield |
|---|---|
| With sulfuric acid In water at 110℃; for 10h; |

-
A
-
87-72-9, 608-45-7, 608-46-8, 608-47-9, 2460-44-8, 6748-95-4, 6763-34-4, 7261-26-9, 7283-06-9, 7283-07-0, 7296-55-1, 7296-56-2, 7296-58-4, 7296-59-5, 7296-60-8, 7296-61-9, 7296-62-0, 7322-30-7, 10257-31-5, 10257-32-6, 10257-33-7, 10257-34-8, 10257-35-9, 19982-83-3, 20242-88-0, 28697-53-2, 36562-42-2, 41546-41-2, 89299-64-9, 107655-34-5, 115794-06-4, 115794-07-5, 130550-15-1, 130606-21-2
D-Xylose

-
B
-
2280-44-6
D-Glucose

-
C
-
10257-28-0
D-Galactose

-
D
-
77-60-1
tigogenin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 110℃; for 4h; |


-
-
77-60-1
tigogenin

| Conditions | Yield |
|---|---|
| With sulfuric acid In ethanol for 2h; Heating; |

| Conditions | Yield |
|---|---|
| Aspergillus niger; |

| Conditions | Yield |
|---|---|
| Aspergillus niger; |
-
-
110124-76-0
(25R)-5α-spirostan-3β-ol 3-O-

-
-
77-60-1
tigogenin

| Conditions | Yield |
|---|---|
| With sulfuric acid at 105℃; for 12h; |
-
-
35959-24-1
(25R)-5α-spirostan-3β-ol 3-O-β-D-galactopyranoside

-
-
77-60-1
tigogenin

| Conditions | Yield |
|---|---|
| With sulfuric acid at 105℃; for 12h; |

-
-
77-60-1
tigogenin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane; water at 100℃; | 7.2 mg |

| Conditions | Yield |
|---|---|
| Hydrogenation; |


| Conditions | Yield |
|---|---|
| Hydrogenation; |


| Conditions | Yield |
|---|---|
| Hydrogenation; |


| Conditions | Yield |
|---|---|
| Hydrogenation; |

-
-
7803-57-8
hydrazine hydrate

-
-
107-21-1
ethylene glycol

-
-
28404-66-2
(25R)-3β-hydroxy-5α-spirostan-23-one

-
-
77-60-1
tigogenin

| Conditions | Yield |
|---|---|
| at 195℃; |


-
-
77-60-1
tigogenin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 37℃; for 192h; |

-
-
77-60-1
tigogenin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 37℃; for 192h; |

-
-
77-60-1
tigogenin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 37℃; for 192h; |

| Conditions | Yield |
|---|---|
| With pyridine; dmap In dichloromethane at 20℃; | 100% |
| With pyridine for 3h; |

| Conditions | Yield |
|---|---|
| With pyridine at 45℃; | 99% |
| With dmap In tetrahydrofuran at 20℃; for 24h; Inert atmosphere; | 96% |
-
-
77-60-1
tigogenin

-
-
151767-11-2
2,3,4,5-tetra-O-benzoyl-D-glucopyranosyl trichloroacetimidate

-
-
885453-92-9
tigogenin 3β-O-(2,3,4,6-tetra-O-benzoyl-β-D-glucopyranoside)

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate; 4 A molecular sieve In dichloromethane at 0 - 20℃; | 97% |

| Conditions | Yield |
|---|---|
| With chromium(VI) oxide; sulfuric acid In acetone at 30℃; for 0.5h; Jones oxidation; | 93% |
| With pyridinium chlorochromate In dichloromethane | 90.4% |
| With sodium acetate; silica gel; pyridinium chlorochromate In dichloromethane at 0 - 20℃; for 15h; | 87% |
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; 2,3-dicyano-5,6-dichloro-p-benzoquinone In dichloromethane at 0 - 20℃; for 24h; Solvent; | 80.2% |
| With oxalyl dichloride; dimethyl sulfoxide; triethylamine 1) CH2Cl2, THF, -78 deg C, 30 min, 2a) -78 deg C, 40 min, 2b) up to r.t.; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| Stage #1: tigogenin With acetic anhydride In acetic acid at 200℃; for 1h; Stage #2: With dihydrogen peroxide; sodium phosphotungstate In water; butan-1-ol at 80℃; for 2h; Stage #3: With sodium hydroxide In water; butan-1-ol for 2h; Product distribution / selectivity; Heating / reflux; | A 92% B 80% |
| Stage #1: tigogenin With acetic anhydride In acetic acid at 200℃; for 1h; Stage #2: With sulfuric acid; dihydrogen peroxide; sodium molybdate In water; N,N-dimethyl-formamide at 80℃; for 2h; Stage #3: With potassium hydroxide In water; butan-1-ol at 80℃; for 2h; Product distribution / selectivity; | A 90% B 84% |
| Stage #1: tigogenin With acetic anhydride In acetic acid at 200℃; for 1h; Stage #2: With dihydrogen peroxide; H7[(PMo2O7)6]*xH2O In water; acetic anhydride; acetic acid at 80℃; for 2h; Stage #3: With potassium hydroxide In ethanol; water for 2h; Product distribution / selectivity; Heating / reflux; | A 86% B 80% |
-
-
77-60-1
tigogenin

-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
156590-26-0
(3β,5α,25R)-3-trimethylsilyloxyspirostane

| Conditions | Yield |
|---|---|
| In acetonitrile for 6h; Heating; | 91% |

| Conditions | Yield |
|---|---|
| Stage #1: tigogenin With sulfuric acid; tetrabutylammomium bromide; iodine; acetic acid; trifluoroacetic acid In dichloromethane at 50℃; for 1h; Stage #2: With dihydrogen peroxide In dichloromethane; water at 0 - 20℃; Stage #3: With water; lithium hydroxide In tetrahydrofuran; dichloromethane for 1h; Reflux; | 91% |
| Stage #1: tigogenin With peroxyacetic acid; sulfuric acid; iodine; acetic acid In acetic anhydride at 20℃; for 24h; Stage #2: With potassium hydroxide In ethanol at 20℃; for 12h; | 89% |
| Stage #1: tigogenin With peroxyacetic acid; sulfuric acid; iodine In acetic acid at 20℃; Stage #2: With sodium hydroxide In water; acetic acid at 20℃; pH=13; | 85% |
| Stage #1: tigogenin With sulfuric acid; iodine; acetic acid at 20℃; for 0.166667h; Stage #2: With peracetic acid; acetic acid for 14h; Stage #3: With sodium hydroxide at 20℃; pH=13; | 85% |
-
-
77-60-1
tigogenin

-
-
661460-75-9
(25R)-5α-cholestane-3β,16β,26-triol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; zinc In ethanol for 0.5h; Heating; | 90% |
-
-
77-60-1
tigogenin

-
-
149625-81-0
(1aS,3R,3aS,6aS,6bR)-3-Triisopropylsilanyloxymethyl-tetrahydro-1,2,4,6-tetraoxa-cyclopropa[e]inden-5-one

| Conditions | Yield |
|---|---|
| With zinc(II) chloride In tetrahydrofuran | 89% |
| With zinc(II) chloride In tetrahydrofuran; diethyl ether at -78 - 25℃; for 12h; Yield given; |
-
-
77-60-1
tigogenin

-
-
81058-27-7
2,3,4,6-tetra-O-pivaloyl-α-D-glucopyranosyl bromide

-
-
100102-42-9
<(25R)-5α-Spirostan-3β-yl>-2,3,4,6-tetra-O-pivaloyl-β-D-glucopyranosid

| Conditions | Yield |
|---|---|
| With molecular sieve; silver carbonate In diethyl ether for 24h; Ambient temperature; | 88% |
-
-
77-60-1
tigogenin

-
-
149707-75-5
2,3,4,6-tetra-O-benzoyl-D-glucopyranosyl trichloroacetimidate

-
-
885453-92-9
tigogenin 3β-O-(2,3,4,6-tetra-O-benzoyl-β-D-glucopyranoside)

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In dichloromethane at 20℃; for 1h; Inert atmosphere; Molecular sieve; | 87% |
| Stage #1: tigogenin; 2,3,4,6-tetra-O-benzoyl-D-glucopyranosyl trichloroacetimidate In dichloromethane for 1h; Molecular sieve; Inert atmosphere; Stage #2: With trimethylsilyl trifluoromethanesulfonate In dichloromethane at 0℃; for 3h; Inert atmosphere; | 65% |

| Conditions | Yield |
|---|---|
| Stage #1: tigogenin With boron trifluoride diethyl etherate; 3-chloro-benzenecarboperoxoic acid In dichloromethane at 10℃; for 2h; Baeyer-Villiger oxidation; Stage #2: With sodium hydroxide In methanol Ring cleavage; Further stages.; | 85% |
| With potassium sulfate; dipotassium peroxodisulfate; sulfuric acid Erhitzen des Reaktionsprodukts mit aethanol. KOH; |
-
-
77-60-1
tigogenin


-
-
1269649-29-7
tigogenyl 2,3,4-tri-O-benzoyl-β-D-xylopyranosyl-(1->3)-[2,3,4-tri-O-acetyl-α-L-rhamnopyransyl-(1->2)]-4,6-di-O-benzylidene-β-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In dichloromethane at 0℃; for 0.666667h; Inert atmosphere; | 81% |
-
-
77-60-1
tigogenin

-
-
440-03-9
2,3,6-tri-O-acetyl-4-O-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl)-β-D-glucopyranosyl fluoride

-
-
101490-77-1
tigogenin cellobioside heptaacetate (β-anomer)

| Conditions | Yield |
|---|---|
| In toluene at 112℃; for 6.5h; | 75% |

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve; boron trifluoride diethyl etherate In dichloromethane at -10℃; for 0.333333h; | 73% |
-
-
77-60-1
tigogenin

-
-
321125-08-0
2-O-(4-methoxybenzoyl)-3,4-di-O-triethylsilyl-β-D-xylopyranosyl-(1→3)-2-O-acetyl-4-O-triethylsilyl-α,β-L-arabinopyranosyl trichloroacetimidate

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate; 4 Angstroem MS In dichloromethane at -20℃; | 71% |
-
-
77-60-1
tigogenin

-
-
615570-19-9
3,6-di-O-benzoylated-isopropyl-β-D-1-thiogalactopyranoside

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide; trimethylsilyl trifluoromethanesulfonate In dichloromethane at -40℃; for 0.666667h; Inert atmosphere; | 71% |

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve; boron trifluoride diethyl etherate In dichloromethane at -10℃; for 0.333333h; | 70% |
-
-
77-60-1
tigogenin

-
-
130948-37-7
C53H52NO8PS

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve; boron trifluoride diethyl etherate In dichloromethane at -23℃; for 8h; | 70% |

| Conditions | Yield |
|---|---|
| With diphenyl diselenide; 3-iodylbenzoic acid In toluene for 7h; Reflux; | 70% |

| Conditions | Yield |
|---|---|
| Multistep reaction.; | 69.5% |
| Stage #1: tigogenin; acetic anhydride With pyridine for 1h; Reflux; Stage #2: titanium tetrachloride In acetic anhydride at 100℃; Reflux; Stage #3: With chromium(VI) oxide; acetic acid In water; acetone at 15 - 18℃; | 69.5% |
| Stage #1: tigogenin; acetic anhydride In pyridine for 1h; Reflux; Stage #2: titanium tetrachloride In pyridine at 100℃; for 2h; Reflux; Stage #3: With chromium(VI) oxide; sodium acetate; acetic acid In pyridine; water; acetone at 15 - 18℃; for 1h; | 69.5% |
| Stage #1: tigogenin; acetic anhydride With pyridine; ammonium chloride at 125 - 135℃; for 9h; Stage #2: With chromium(VI) oxide; water; acetic acid In 1,2-dichloro-ethane at 7 - 20℃; | 55% |
| Stage #1: tigogenin; acetic anhydride With acetic acid at 208 - 225℃; under 3600.36 - 4875.49 Torr; for 1.08333h; Stage #2: With chromic acid at 12 - 98℃; for 0.75h; Temperature; |
-
-
77-60-1
tigogenin

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide; trimethylsilyl trifluoromethanesulfonate In dichloromethane at -40℃; for 0.5h; Inert atmosphere; | 65% |
-
-
77-60-1
tigogenin

-
-
773145-61-2
2,3,4,6-tetra-O-benzoyl-β-D-glucopyranosyl-(1->4)-2,3,6-tri-O-benzoyl-α-D-glucopyranosyl trichloroacetimidate

-
-
1352548-84-5
3-O-[2,3,4,6-tetra-O-benzoyl-β-D-glucopyranosyl-(1→4)-2,3,6-tri-O-benzoyl-β-D-glucopyranosyl]tigogenin

| Conditions | Yield |
|---|---|
| Stage #1: tigogenin; 2,3,4,6-tetra-O-benzoyl-β-D-glucopyranosyl-(1->4)-2,3,6-tri-O-benzoyl-α-D-glucopyranosyl trichloroacetimidate In dichloromethane for 1h; Molecular sieve; Inert atmosphere; Stage #2: With trimethylsilyl trifluoromethanesulfonate In dichloromethane at 0℃; for 3h; Inert atmosphere; | 61% |
-
-
77-60-1
tigogenin

-
-
343980-32-5
(O-2,3,4,6-tetra-O-benzoyl-α-D-glucopyranosyl-(1→4)-2,3,6-tri-O-benzoyl-α-D-glucopyranosyl)-trichloroacetimidate

-
-
1352548-83-4
3-O-[2,3,4,6-tetra-O-benzoyl-α-D-glucopyranosyl-(1→4)-2,3,6-tri-O-benzoyl-β-D-glucopyranosyl]tigogenin

| Conditions | Yield |
|---|---|
| Stage #1: tigogenin; (O-2,3,4,6-tetra-O-benzoyl-α-D-glucopyranosyl-(1→4)-2,3,6-tri-O-benzoyl-α-D-glucopyranosyl)-trichloroacetimidate In dichloromethane for 1h; Molecular sieve; Inert atmosphere; Stage #2: With trimethylsilyl trifluoromethanesulfonate In dichloromethane at 0℃; for 3h; Inert atmosphere; | 58% |

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride In acetic acid for 2.5h; Ring cleavage; | 55% |
Tigogenin Specification
The CAS registry number of Tigogenin is 77-60-1. The systematic name is (3β,5α,25R)-spirostan-3-ol. Its EINECS registry number is 201-041-9. In addition, the molecular formula is C27H44O3 and the molecular weight is 416.64. It belongs to the class of Miscellaneous Natural Products.
Physical properties about this chemical are: (1)ACD/LogP: 6.21; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 6.21; (4)ACD/LogD (pH 7.4): 6.21; (5)ACD/BCF (pH 5.5): 30645.03; (6)ACD/BCF (pH 7.4): 30645.03; (7)ACD/KOC (pH 5.5): 56613.64; (8)ACD/KOC (pH 7.4): 56613.64; (9)#H bond acceptors: 3; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 27.69 Å2; (13)Index of Refraction: 1.552; (14)Molar Refractivity: 119.65 cm3; (15)Molar Volume: 374.3 cm3; (16)Polarizability: 47.43 ×10-24cm3; (17)Surface Tension: 43.8 dyne/cm; (18)Density: 1.11 g/cm3; (19)Flash Point: 266.2 °C; (20)Enthalpy of Vaporization: 90.78 kJ/mol; (21)Boiling Point: 516.6 °C at 760 mmHg; (22)Vapour Pressure: 7.83E-13 mmHg at 25°C.
Uses of Tigogenin: it can be used as raw materials of dehydro-methyl testosterone and rehabilitation dragons. And it can be used to get (20S)-5a-pregnanetriol-(3b.16b.20). This reaction is a kind of Baeyer-Villiger oxidation reaction. And it will need reagents MCPBA, BF3*Et2O and NaOH and solvents CH2Cl2 and methanol. The reaction time is 2 hours at reaction temperature of 10 °C. The yield is about 85%.
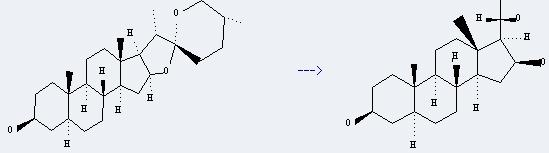
You can still convert the following datas into molecular structure:
(1)SMILES: O1[C@@H]4[C@H]([C@@H]([C@]12OC[C@@H](CC2)C)C)[C@@]5(C)CC[C@@H]3[C@@]6(C)CC[C@H](O)C[C@@H]6CC[C@H]3[C@@H]5C4
(2)InChI: InChI=1/C27H44O3/c1-16-7-12-27(29-15-16)17(2)24-23(30-27)14-22-20-6-5-18-13-19(28)8-10-25(18,3)21(20)9-11-26(22,24)4/h16-24,28H,5-15H2,1-4H3/t16-,17+,18+,19+,20-,21+,22+,23+,24+,25+,26+,27-/m1/s1
(3)InChIKey: GMBQZIIUCVWOCD-MFRNJXNGBQ
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LDLo | intraperitoneal | 10mg/kg (10mg/kg) | "Toxins of Animal and Plant Origin, Proceedings International Symposium, 2nd, 1970," de Vries A., and E. Kochva, eds., 3 vols., New York, Gordon and Breach Science Pub., 1971-73Vol. 2, Pg. 597, 1972. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View