-
Name
Trifluoroacetamide
- EINECS 206-559-9
- CAS No. 354-38-1
- Article Data67
- CAS DataBase
- Density 1.435 g/cm3
- Solubility water: 460 g/L(20 °C)
- Melting Point 65-70 °C(lit.)
- Formula C2H2F3NO
- Boiling Point 162.5 °C at 760 mmHg
- Molecular Weight 113.039
- Flash Point 52.1 °C
- Transport Information
- Appearance White to pale yellow or beige crystalline powder
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2,2,2-Trifluoroacetamide;NSC 9449;
- PSA 43.09000
- LogP 0.73430
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogenchloride; triethylamine In ethanol; ethyl acetate | 100% |
| With diethyl ether; ammonia | |
| With methanol; ammonia |

| Conditions | Yield |
|---|---|
| With methylamine at 150℃; under 750.075 Torr; for 10h; Gas phase; | 99.1% |
-
-
3888-00-4
nitropentafluoroacetone

-
A
-
1493-05-6
difluoro-nitro-methane

-
B
-
354-38-1
2,2,2-trifluoroacetamide

| Conditions | Yield |
|---|---|
| With ammonia in bomb tube; | A 14% B 99% |
| With NH3 in bomb tube; | A 14% B 99% |
-
-
52225-57-7
2,2,2-trifluoro-N-{2,2,2-trifluoro-1-(trifluoromethyl) ethylidene}acetamide

-
-
103-69-5
N-ethyl-N-phenylamine

-
A
-
65797-85-5
2-(4-(ethylamino)phenyl)-1,1,1,3,3,3-hexafluoropropan-2-ol

-
B
-
677-71-4
hexafluoroacetone hydrate

-
C
-
354-38-1
2,2,2-trifluoroacetamide

-
D
-
106119-57-7
2,4,4-tris(trifluoromethyl)-1-ethyl-1,4-dihydroquinazoline

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 1440h; Mechanism; | A n/a B n/a C n/a D 87% E n/a |

-
-
89567-94-2
N-trifluoroacetyl-Se-(p-bromophenyl)-Se-methylselenimide

-
A
-
354-38-1
2,2,2-trifluoroacetamide

-
B
-
78238-44-5
methyl (p-bromophenyl)selenium dichloride

| Conditions | Yield |
|---|---|
| With acetyl chloride In chloroform at 20℃; for 3h; | A 55% B 85% |
-
-
84353-59-3
1-(2,2,2-trifluoroacetyl)-3-(E)-styrylurea

-
A
-
354-38-1
2,2,2-trifluoroacetamide

-
B
-
84353-56-0
(E)-N-(2-phenylethenyl)-2,2,2-trifluoroacetamide

| Conditions | Yield |
|---|---|
| In trifluoroacetic acid at 230℃; for 0.333333h; | A 69% B 22% |
-
-
84353-55-9
1-(2,2,2-trifluoroacetyl)-3-<2-(2,2,2-trifluoroacetyloxy)-2-phenylethyl>urea

-
A
-
354-38-1
2,2,2-trifluoroacetamide

-
B
-
84353-56-0
(E)-N-(2-phenylethenyl)-2,2,2-trifluoroacetamide

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 230℃; for 0.5h; | A 69% B 19% |
-
-
84353-59-3
1-(2,2,2-trifluoroacetyl)-3-(E)-styrylurea

-
-
354-38-1
2,2,2-trifluoroacetamide

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 230℃; for 0.333333h; | 68% |
-
-
126-81-8
dimedone

-
-
77571-23-4, 78780-20-8, 78780-21-9
N-(trifluoroacetyl)-di(p-methoxyphenyl)tellurimide

-
A
-
354-38-1
2,2,2-trifluoroacetamide

-
B
-
57857-72-4
di(p-metoxyphenyl)telluroniodimedonide

| Conditions | Yield |
|---|---|
| In chloroform Ambient temperature; overnight; | A 58% B 67% |


| Conditions | Yield |
|---|---|
| With zirconium(IV) chloride; ammonium carbamate In toluene at 120℃; for 24h; Inert atmosphere; Molecular sieve; | 67% |
-
-
233257-02-8
[(trifluoroacetyl)imino]tris-(2-methylphenyl)-λ(5)-bismuthane

-
A
-
354-38-1
2,2,2-trifluoroacetamide

-
B
-
10050-08-5
tri-o-tolylbismuth

| Conditions | Yield |
|---|---|
| In benzene 60°C, 48 h; | A n/a B 61% |
-
-
1868-48-0
2,5-bis(trifluoromethyl)-1,3,4-oxadiazole

-
-
513-81-5
2,3-dimethyl-buta-1,3-diene

-
B
-
354-38-1
2,2,2-trifluoroacetamide

-
C
-
100696-00-2
2-(trifluoromethyl)-4,5-dimethylpyridine

| Conditions | Yield |
|---|---|
| at 150℃; for 18h; | A 32% B 15% C 30% |

-
-
944903-98-4
2-trifluoromethyl-5-ethoxycarbonyl-1,3,4-oxadiazole

-
-
513-81-5
2,3-dimethyl-buta-1,3-diene

-
B
-
354-38-1
2,2,2-trifluoroacetamide

-
C
-
100696-00-2
2-(trifluoromethyl)-4,5-dimethylpyridine

| Conditions | Yield |
|---|---|
| at 150℃; for 14h; | A 27% B n/a C n/a |

-
-
359-45-5
N-bromo-trifluoroacetamide

-
A
-
354-38-1
2,2,2-trifluoroacetamide

-
-
78162-82-0, 78174-04-6
(+/-)-trifluoroacetic acid-(trans-2-bromo-cyclohexylamide)

-
-
78162-82-0, 78174-04-6
N-((1R,2S)-2-Bromo-cyclohexyl)-2,2,2-trifluoro-acetamide

| Conditions | Yield |
|---|---|
| With cyclohexene In dichloromethane at 15 - 20℃; for 0.2h; Irradiation; Yields of byproduct given; | A 7% B n/a C n/a |

-
-
359-45-5
N-bromo-trifluoroacetamide

-
-
110-83-8
cyclohexene

-
A
-
354-38-1
2,2,2-trifluoroacetamide

-
-
78162-82-0, 78174-04-6
(+/-)-trifluoroacetic acid-(trans-2-bromo-cyclohexylamide)

-
-
78162-82-0, 78174-04-6
N-((1R,2S)-2-Bromo-cyclohexyl)-2,2,2-trifluoro-acetamide

| Conditions | Yield |
|---|---|
| In dichloromethane at 15 - 20℃; for 0.2h; Irradiation; Yield given. Yields of byproduct given; | A 7% B n/a C n/a |

-
-
83643-84-9
methyl 4,4,4-trifluoroacetoacetate

-
A
-
60-35-5
acetamide

-
B
-
354-38-1
2,2,2-trifluoroacetamide

-
C
-
107638-26-6
3-amino-4,4,4-trifluorobut-2-enoic acid

| Conditions | Yield |
|---|---|
| With ammonia In diethyl ether at 100℃; for 10h; Product distribution; NH3 amount dependence; | A n/a B n/a C 5% |


| Conditions | Yield |
|---|---|
| With ammonia In diethyl ether |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In diethyl ether |
-
-
55982-15-5
N-(trimethylsilyl)trifluoroacetamide

-
-
354-38-1
2,2,2-trifluoroacetamide

| Conditions | Yield |
|---|---|
| With boron trifluoride |

| Conditions | Yield |
|---|---|
| With ammonia In benzene |
-
-
93032-04-3
2,2-Dibromo-4,4,4-trifluoro-3-oxo-butyric acid methyl ester

-
A
-
598-70-9
dibromoacetamide

-
B
-
354-38-1
2,2,2-trifluoroacetamide

| Conditions | Yield |
|---|---|
| With ammonia In diethyl ether at -40 - -10℃; Yield given. Yields of byproduct given; |

| Conditions | Yield |
|---|---|
| With ammonia In diethyl ether at -40 - -10℃; Yield given. Yields of byproduct given; |
-
-
14565-32-3
2,2,2-trifluoroacetyl isocyanate

-
-
2925-25-9
trifluorothioacetic acid

-
A
-
354-38-1
2,2,2-trifluoroacetamide

-
B
-
407-24-9
bistrifluoroacetamide

| Conditions | Yield |
|---|---|
| Yield given; |

-
-
79-14-1
glycolic Acid

-
-
25561-30-2
N,O-bis(trimethylsilyl)trifluoroacetamide

-
A
-
354-38-1
2,2,2-trifluoroacetamide

-
B
-
33581-77-0
trimethylsilyl [(trimethylsilyl)oxy]acetate

-
C
-
55982-15-5
N-(trimethylsilyl)trifluoroacetamide

| Conditions | Yield |
|---|---|
| In hexane; dichloromethane at 60℃; for 0.666667h; Substitution; |

-
-
849585-22-4
LACTIC ACID

-
-
25561-30-2
N,O-bis(trimethylsilyl)trifluoroacetamide

-
A
-
354-38-1
2,2,2-trifluoroacetamide

-
B
-
17596-96-2
propanoic acid,2-[(trimethylsilyl)oxy] trimethylsilyl ester

-
C
-
55982-15-5
N-(trimethylsilyl)trifluoroacetamide

| Conditions | Yield |
|---|---|
| In hexane; dichloromethane at 60℃; for 0.666667h; Substitution; |

-
-
110-94-1
1,5-pentanedioic acid

-
-
25561-30-2
N,O-bis(trimethylsilyl)trifluoroacetamide

-
A
-
354-38-1
2,2,2-trifluoroacetamide

-
B
-
55494-07-0
bis-trimethylsilyl glutarate

-
C
-
55982-15-5
N-(trimethylsilyl)trifluoroacetamide

| Conditions | Yield |
|---|---|
| In hexane; dichloromethane at 60℃; for 0.666667h; Substitution; |

-
-
124-04-9
Adipic acid

-
-
25561-30-2
N,O-bis(trimethylsilyl)trifluoroacetamide

-
A
-
354-38-1
2,2,2-trifluoroacetamide

-
B
-
18105-31-2
Bis(trimethylsilyl) adipate

-
C
-
55982-15-5
N-(trimethylsilyl)trifluoroacetamide

| Conditions | Yield |
|---|---|
| In hexane; dichloromethane at 60℃; for 0.666667h; Substitution; |

-
-
111-14-8
oenanthic acid

-
-
25561-30-2
N,O-bis(trimethylsilyl)trifluoroacetamide

-
A
-
354-38-1
2,2,2-trifluoroacetamide

-
B
-
25435-96-5
l'heptanoate de trimethylsilyle

-
C
-
55982-15-5
N-(trimethylsilyl)trifluoroacetamide

| Conditions | Yield |
|---|---|
| In hexane; dichloromethane at 60℃; for 0.666667h; Substitution; |

-
-
124-07-2
Octanoic acid

-
-
25561-30-2
N,O-bis(trimethylsilyl)trifluoroacetamide

-
A
-
354-38-1
2,2,2-trifluoroacetamide

-
B
-
55982-15-5
N-(trimethylsilyl)trifluoroacetamide

| Conditions | Yield |
|---|---|
| In hexane; dichloromethane at 60℃; for 0.666667h; Substitution; |

-
-
141-82-2
malonic acid

-
-
25561-30-2
N,O-bis(trimethylsilyl)trifluoroacetamide

-
A
-
354-38-1
2,2,2-trifluoroacetamide

-
B
-
18457-04-0
bis(trimethylsilyl) malonate

-
C
-
55982-15-5
N-(trimethylsilyl)trifluoroacetamide

| Conditions | Yield |
|---|---|
| In hexane; dichloromethane at 60℃; for 0.666667h; Substitution; |


-
-
354-38-1
2,2,2-trifluoroacetamide

-
-
152071-56-2
N-(trimethylstannyl)-2,2,2-trifluoroacetamide

| Conditions | Yield |
|---|---|
| In benzene byproducts: water; absence of moisture; equimolar amts., refluxing for 3 h (distn. off of water); solvent removal (vac.); | 100% |
-
-
354-38-1
2,2,2-trifluoroacetamide

-
-
286367-11-1
2-(4-(trifluoromethyl)phenyl)-4,5-dihydrooxazole

| Conditions | Yield |
|---|---|
| With silver hexafluoroantimonate; dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; [bis(acetoxy)iodo]benzene In dichloromethane at 40℃; for 16h; Inert atmosphere; regioselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| With silver hexafluoroantimonate; dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; [bis(acetoxy)iodo]benzene In dichloromethane at 40℃; for 16h; Inert atmosphere; regioselective reaction; | 100% |
-
-
13676-94-3
2-(4-methoxyphenyl)-4,5-dihydrooxazole

-
-
354-38-1
2,2,2-trifluoroacetamide

| Conditions | Yield |
|---|---|
| With silver hexafluoroantimonate; dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; [bis(acetoxy)iodo]benzene In dichloromethane at 40℃; for 16h; Inert atmosphere; regioselective reaction; | 100% |
| With silver hexafluoroantimonate; dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; [bis(acetoxy)iodo]benzene In dichloromethane at 40℃; for 16h; Schlenk technique; Inert atmosphere; Sealed tube; | 100% |
-
-
354-38-1
2,2,2-trifluoroacetamide

| Conditions | Yield |
|---|---|
| With dirhodium tetraacetate; [bis(acetoxy)iodo]benzene; magnesium oxide In dichloromethane for 3h; Reflux; | 100% |
-
-
354-38-1
2,2,2-trifluoroacetamide

| Conditions | Yield |
|---|---|
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; sodium hydride In tetrahydrofuran at 20℃; for 2h; | 100% |

-
-
354-38-1
2,2,2-trifluoroacetamide

| Conditions | Yield |
|---|---|
| Stage #1: 2,2,2-trifluoroacetamide With potassium hexamethylsilazane In tetrahydrofuran at -78℃; for 0.5h; Inert atmosphere; Stage #2: (S)-2-methyl-1-[(2-nitrophenyl)sulfonyl]aziridine In tetrahydrofuran; dichloromethane at 20℃; for 18h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With copper(II) oxide at 400℃; under 750.075 Torr; for 10h; Gas phase; | 99.1% |
| With pyridine; pivaloyl chloride; trifluoroacetic acid at 20℃; for 5h; Product distribution / selectivity; | 92% |
| With phosphorus pentoxide Heating; | 90% |
-
-
354-38-1
2,2,2-trifluoroacetamide

-
-
91043-70-8
N-Methoxymethylmonoaza-15-crown-5 ether

-
-
127846-31-5
2,2,2-Trifluoro-N-(1,4,7,10-tetraoxa-13-aza-cyclopentadec-13-ylmethyl)-acetamide

| Conditions | Yield |
|---|---|
| In tetrachloromethane Heating; | 99% |
-
-
354-38-1
2,2,2-trifluoroacetamide

-
-
586-77-6
4-bromo-N,N-dimethylaniline

-
-
41116-22-7
2,2,2-trifluoro-(4-N,N-dimethyphenyl)acetamide

| Conditions | Yield |
|---|---|
| With potassium carbonate; N,N`-dimethylethylenediamine; copper(l) iodide In 1,4-dioxane at 75℃; | 99% |

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium(0) chloroform complex; 1,1'-bis(diphenylphosphino)ferrocene In toluene at 100℃; for 3h; Inert atmosphere; optical yield given as %de; stereoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium(0) chloroform complex; 1,1'-bis(diphenylphosphino)ferrocene In toluene at 100℃; for 3h; Inert atmosphere; optical yield given as %de; stereoselective reaction; | 99% |
-
-
354-38-1
2,2,2-trifluoroacetamide

-
-
220466-11-5
1-isocyanato-2-(2-phenylethynyl)benzene

-
-
1152309-90-4
C17H11F3N2O2

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium(0) chloroform complex; 1,1'-bis(diphenylphosphino)ferrocene In toluene at 100℃; for 3h; Inert atmosphere; optical yield given as %de; stereoselective reaction; | 99% |
-
-
4117-09-3
7-bromo-hept-1-ene

-
-
354-38-1
2,2,2-trifluoroacetamide

-
-
1268372-73-1
2,2,2-trifluoro-N-(hept-6-en-1-yl)acetamide

| Conditions | Yield |
|---|---|
| Stage #1: 2,2,2-trifluoroacetamide With sodium hydride In N,N-dimethyl-formamide at 0℃; Inert atmosphere; Stage #2: 7-bromo-hept-1-ene In N,N-dimethyl-formamide at 50℃; for 3h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,2,2-trifluoroacetamide With sodium hydride In N,N-dimethyl-formamide at 0℃; Inert atmosphere; Stage #2: bromopentene In N,N-dimethyl-formamide at 50℃; for 3h; Inert atmosphere; | 99% |
| Stage #1: 2,2,2-trifluoroacetamide With sodium hydride In N,N-dimethyl-formamide; paraffin oil at 20℃; for 2h; Stage #2: bromopentene In N,N-dimethyl-formamide; paraffin oil at 50℃; for 21h; | 63% |
-
-
354-38-1
2,2,2-trifluoroacetamide

| Conditions | Yield |
|---|---|
| Stage #1: 2,2,2-trifluoroacetamide With potassium hexamethylsilazane In tetrahydrofuran at -78℃; for 0.5h; Inert atmosphere; Stage #2: (S)-2-benzyl-1-((2-nitrophenyl)sulfonyl)aziridine In tetrahydrofuran; dichloromethane at 20℃; for 18h; Inert atmosphere; | 99% |
-
-
354-38-1
2,2,2-trifluoroacetamide

-
-
89384-43-0
[1-(1-Cyano-3-methyl-butylcarbamoyl)-2-phenyl-ethyl]-carbamic acid methyl ester

-
-
89384-44-1
{1-[4-Isobutyl-5-(2,2,2-trifluoro-acetylamino)-oxazol-2-yl]-2-phenyl-ethyl}-carbamic acid methyl ester

| Conditions | Yield |
|---|---|
| trifluoroacetic acid | 98% |
-
-
354-38-1
2,2,2-trifluoroacetamide

-
-
89384-49-6, 150957-49-6
Cbz-l-Leu-dl-Phe-N-benzyl amide

-
-
87783-99-1
2-<1(S)-(carbobenzyloxyamino)-3-methylbutyl>-4-benzyl-5-(N-benzyltrifluoroacetamido)oxazole

| Conditions | Yield |
|---|---|
| trifluoroacetic acid In dichloromethane | 98% |
-
-
354-38-1
2,2,2-trifluoroacetamide

-
-
2695-47-8
6-Bromo-1-hexene

-
-
183439-11-4
2,2,2-trifluoro-N-(hex-5-en-1-yl)acetamide

| Conditions | Yield |
|---|---|
| Stage #1: 2,2,2-trifluoroacetamide With sodium hydride In N,N-dimethyl-formamide at 0℃; Inert atmosphere; Stage #2: 6-Bromo-1-hexene In N,N-dimethyl-formamide at 50℃; for 3h; Inert atmosphere; | 98% |
| With sodium hydride 1) DMF, 0.5 h; 2) DMF, 80 deg C, 10 h; Yield given. Multistep reaction; |
-
-
354-38-1
2,2,2-trifluoroacetamide

-
-
139-66-2
diphenyl sulfide

-
-
87446-53-5
S,S-diphenyl-N-(trifluoroacetyl)sulfilimine

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; magnesium oxide; dirhodium tetraacetate In dichloromethane at 20℃; for 6h; | 98% |
| With rhodium(II) acetate dimer; [bis(acetoxy)iodo]benzene; magnesium oxide In dichloromethane at 20℃; for 18h; Inert atmosphere; |
-
-
354-38-1
2,2,2-trifluoroacetamide

-
-
92-66-0
4-bromo-1,1'-biphenyl

-
-
347-37-5
2,2,2-trifluoro-4'-phenylacetanilide

| Conditions | Yield |
|---|---|
| With potassium carbonate; N,N`-dimethylethylenediamine; copper(l) iodide In 1,4-dioxane at 75℃; | 98% |
-
-
354-38-1
2,2,2-trifluoroacetamide

-
-
1352227-18-9
(3R)-4-(2-chloro-6-[(methylsulfinyl)methyl]-4-pyrimidinyl)-3-methylmorpholine

| Conditions | Yield |
|---|---|
| With rhodium(II) acetate; [bis(acetoxy)iodo]benzene; magnesium oxide In dichloromethane at 20℃; for 16h; Inert atmosphere; | 98% |
| With [bis(acetoxy)iodo]benzene; magnesium oxide; dirhodium tetraacetate In dichloromethane at 20℃; for 96h; | 82% |
| With dirhodium tetraacetate; [bis(acetoxy)iodo]benzene; magnesium oxide In dichloromethane at 20℃; for 96h; | 82% |
| With rhodium(II) acetate dimer; [bis(acetoxy)iodo]benzene In dichloromethane at 20℃; for 36h; Reagent/catalyst; | 64.82% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; [methanesulfonato2-dicyclohexylphosphino-2-(N,N-dimethylamino)biphenyl(2′-amino-1,1′-biphenyl-2-yl)palladium(II)] In toluene at 100℃; for 12h; Inert atmosphere; Sealed tube; Schlenk technique; | 98% |
-
-
354-38-1
2,2,2-trifluoroacetamide

| Conditions | Yield |
|---|---|
| Stage #1: 2,2,2-trifluoroacetamide With potassium hexamethylsilazane In tetrahydrofuran at -78℃; for 0.5h; Inert atmosphere; Stage #2: (S)-2-isobutyl-1-[(2-nitrophenyl)sulfonyl]aziridine In tetrahydrofuran; dichloromethane at 20℃; for 18h; Inert atmosphere; | 98% |
-
-
354-38-1
2,2,2-trifluoroacetamide

| Conditions | Yield |
|---|---|
| With tris(2,2'-bipyridyl)ruthenium dichloride; sodium carbonate In 1,2-dichloro-ethane at 20℃; for 8h; Irradiation; | 98% |
-
-
354-38-1
2,2,2-trifluoroacetamide

-
-
67082-95-5
3-(3-phenylpropyl-2-yn-1-ylidene)-2,4-pentanedione

| Conditions | Yield |
|---|---|
| With tris(2,2'-bipyridyl)ruthenium dichloride; sodium carbonate In 1,2-dichloro-ethane at 20℃; for 8h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Irradiation; | 98% |
-
-
354-38-1
2,2,2-trifluoroacetamide

-
-
120758-24-9
1-benzyloxy-3-tosyloxypropane

-
-
185854-00-6
N-(3-Benzyloxypropyl)-2,2,2-trifluoroacetamide

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; sodium iodide In tetrahydrofuran for 4h; | 97% |
Trifluoroacetamide Chemical Properties
IUPAC Name: 2,2,2-Trifluoroacetamide
Following is the structure of Trifluoroacetamide (CAS NO.354-38-1):
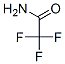
Molecular Formula: C2H2F3NO
Molecular Weight: 113.04g/mol
Mol File: 354-38-1.mol
EINECS: 206-559-9
Melting Point: 65-70 °C(lit.)
Boiling point: 162.5 °C(lit.)
Storage Temperature: Store at RT.
Flash Point: 162-164 °C
Water Solubility: 460 g/L (20 °C)
Surface Tension: 21.4 dyne/cm
Enthalpy of Vaporization: 39.91 kJ/mol
Vapour Pressure: 2.16 mmHg at 25°C
XLogP3-AA: 0.1
H-Bond Donor: 1
H-Bond Acceptor: 4
Appearance of Trifluoroacetamide (CAS NO.354-38-1): White to pale yellow or beige crystalline powder
Product Categories of Trifluoroacetamide (CAS NO.354-38-1): Amides; Carbonyl Compounds; Organic Building Blocks
Canonical SMILES: C(=O)(C(F)(F)F)N
InChI: InChI=1S/C2H2F3NO/c3-2(4,5)1(6)7/h(H2,6,7)
InChIKey: NRKYWOKHZRQRJR-UHFFFAOYSA-N
Trifluoroacetamide Safety Profile
Safety Information of Trifluoroacetamide (CAS NO.354-38-1):
Hazard Codes: Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36-37/39
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
S37/39:Wear suitable gloves and eye/face protection.
Trifluoroacetamide Specification
Trifluoroacetamide , its CAS NO. is 354-38-1, the synonyms are 2,2,2-Trifluoroacetamide ; Acetamide, 2,2,2-trifluoro- ; 2,2,2-Trifluoro-acetamide .
Related Products
- Trifluoroacetamide
- 35438-57-4
- 35439-70-4
- 3544-24-9
- 3544-25-0
- 35444-44-1
- 35445-70-6
- 35446-36-7
- 35447-68-8
- 35447-75-7
- 35447-78-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View