-
Name
Uranium hexafluoride
- EINECS 232-028-6
- CAS No. 7783-81-5
- Article Data252
- CAS DataBase
- Density d420.7 5.09; d70 (liq) 3.595
- Solubility soluble liquid Cl2, Br2; gives dark red fuming solution with nitrobenzene; soluble CCl4, CH3Cl [MER06]
- Melting Point 64.8°
- Formula F6U
- Boiling Point 56.5°C (estimate)
- Molecular Weight 352.019
- Flash Point
- Transport Information
- Appearance
- Safety Radioactive poison. A corrosive irritant to skin, eyes, and mucous membranes. Violent reaction with hydroxy compounds (e.g., ethanol, water). Vigorous reaction with aromatic hydrocarbons (e.g., benzene, toluene, xylene). When heated to decomposition it emits toxic fumes of F−. See also FLUORIDES and URANIUM.
- Risk Codes
-
Molecular Structure
- Hazard Symbols Highly corrosive; radiation risk.
- Synonyms [Uf6];
- PSA 0.00000
- LogP -17.97600
Uranium Fluoride (Fissile) Chemical Properties
Chemistry informtion about Uranium Fluoride (Fissile) (CAS NO.7783-81-5) is:
IUPAC Name: Hexafluorouranium
Synonyms: Uranium Hexafluoride ; Uranium Hexafluoride: (Uranium Fluoride) ; Uranium Hexafluoride, Fissile ; Hexafluorouran(Vi) ; Uran(Vi)Hexafluoride ; Uranium(Vi) Fluoride ; Uranium(Vi)Fluoride(Lowspecificactivity) ; Uranium(Vi)Fluoride(Fissile)
MF: F6U
MW: 352.0193292
EINECS: 232-028-6
Density: 5.09 g/cm3
Melting point: 64.8 °C
Boiling point: 56.5 °C
Following is the molecular structure of Uranium Fluoride (Fissile) (CAS NO.7783-81-5) is:
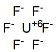
Uranium Fluoride (Fissile) Uses
Uranium Fluoride (Fissile) (CAS NO.7783-81-5) can be used to make fuel for nuclear power plants.
Uranium Fluoride (Fissile) Safety Profile
Radioactive poison. A corrosive irritant to skin, eyes, and mucous membranes. Violent reaction with hydroxy compounds (e.g., ethanol, water). Vigorous reaction with aromatic hydrocarbons (e.g., benzene, toluene, xylene). When heated to decomposition it emits toxic fumes of F−. See also FLUORIDES and URANIUM.
Uranium Fluoride (Fissile) Standards and Recommendations
OSHA PEL: TWA Soluble Compounds: 0.05 mg(U)/m3
ACGIH TLV: TWA 0.2 mg(U)/m3; STEL 0.6 mg(U)/m3; 2.5 mg(F)/m3
DOT Classification: 7; Label: RADIOACTIVE, Corrosive
Uranium Fluoride (Fissile) Analytical Methods
Usually uranium dioxide and hydrogen fluoride at 500°C when the reaction, first obtained uranium tetrafluoride; further with the fluoride in the 300 ~ 350 °C or when the reaction was. Fluoride with fluorine gas uranium tetrafluoride is the most important uranium hexafluoride industrial preparation method, which consumes a minimum of fluorine gas. Is the only volatile compounds of uranium compounds, the gas diffusion method, ultra-centrifugation separation and enrichment of uranium 235 and uranium-238 is the most suitable working fluid, in the atomic energy industry has very important significance. It is the only stable gaseous uranium compounds, widely used in uranium isotope separation plant feeding. UF4+F2→UF6
Uranium Fluoride (Fissile) Specification
Uranium Fluoride (Fissile) (CAS NO.7783-81-5) is a colorless volatile radioactive crystalline solid. It is highly toxic,corrosive and radioactive. It emits high energy rays which may be harmful and are detectable only by special instruments. Chemically irritates skin, eyes and mucous membranes. It in which the uranium has been depleted of the isotope U-235. Naturally occurring uranium contains 0.7% U-235 and 99.3% U-238 (lower radioactivity). Thus, a depleted uranium material with some U-235 removed by the enrichment process is less radioactive. Emits fumes of highly toxic metallic uranium and uranium fluorides when heated to decomposition [Lewis, 3rd ed., 1993, p. 1301]. Reacts vigorously with aromatic hydrocarbons (benzene, toluene, xylenes), undergoes a violent reaction with water or alcohols (methanol, ethanol) [Bretherick, 5th ed., 1995, p. 1439]. Reacts with most metals. Radiation presents minimal risk to transport workers, emergency response personnel and the public during transportation accidents. Packaging durability increases as potential radiation and criticality hazards of the content increase. Chemical hazard greatly exceeds radiation hazard. Substance reacts with water and water vapor in air to form toxic and corrosive hydrogen fluoride gas and an extremely irritating and corrosive, white-colored, water-soluble residue. If inhaled, may be fatal. Direct contact causes burns to skin, eyes, and respiratory tract. Low-level radioactive material; very low radiation hazard to people. Runoff from control of cargo fire may cause low-level pollution. It does not burn. The material may react violently with fuels. Containers in protective overpacks (horizontal cylindrical shape with short legs for tie-downs), are identified with "AF", "B(U)F" or "H(U)" on shipping papers or by markings on the overpacks. They are designed and evaluated to withstand severe conditions including total engulfment in flames at temperatures of 800°C (1475°F) for a period of 30 minutes. Bare filled cylinders, identified with UN2978 as part of the marking (may also be marked H(U) or H(M)), may rupture in heat of engulfing fire; bare empty (except for residue) cylinders will not rupture in fires. Radioactivity does not change flammability or other properties of materials.
Related Products
- Uranium
- URANIUM AZIDE PENTACHLORIDE
- Uranium boride (UB2)
- Uranium bromide (UBr<sub>3</sub>)(7CI,8CI,9CI)
- Uranium carbide
- Uranium dicarbide
- Uranium fluoride
- Uranium Fluoride (Fissile)
- Uranium iodide (UI<sub>4</sub>)
- Uranium Nitrate
- 7783-82-6
- 7783-83-7
- 7783-85-9
- 7783-86-0
- 7783-89-3
- 7783-90-6
- 7783-92-8
- 7783-93-9
- 7783-95-1
- 7783-96-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View