-
Name
DEOXYNIVALENOL
- EINECS 200-835-2
- CAS No. 51481-10-8
- Article Data8
- CAS DataBase
- Density 1.48g/cm3
- Solubility
- Melting Point 151-153 C
- Formula C15H20 O6
- Boiling Point 543.9°Cat760mmHg
- Molecular Weight 296.32
- Flash Point 206.9°C
- Transport Information
- Appearance
- Safety Poison by ingestion, subcutaneous, and intraperitoneal routes. An experimental teratogen. Experimental reproductive effects. A skin irritant. When heated to decomposition it emits acrid smoke and fumes.
- Risk Codes R25; R68/20/21/22; R67; R66; R36; R20/21/22; R11; R36/37/38
-
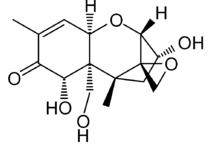
Molecular Structure
-
Hazard Symbols
 T,
T, Xn,
Xn, F,
F, Xi
Xi
- Synonyms Spiro[2,5-methano-1-benzoxepin-10,2'-oxirane],trichothec-9-en-8-one deriv.; 4-Deoxynivalenol; 4-Desoxynivalenol; DON;Dehydronivalenol; Deoxynivalenol; NSC 269144; Vomitoxin
- PSA 99.52000
- LogP -0.83770
VOMITOXIN Chemical Properties
CBNumber: CB0391546
Chemical Name: (3α,7α)-3,7,15-trihydroxy-12,13-epoxytrichothec-9-en-8-one
Other names: DEOXYNIVALENO;Vomitoxin;DON;DEOXYNIVALENOL;3ALPHA,7ALPHA,15-TRIHYDROXY-12,13-EPOXYTRICHOTHEC-9-EN-8-ONE;VOMITOXIN;12,13-epoxy-3,7,15-trihydroxy-,(3-alpha,7-alpha)-trichothec-9-en-8-on;12,13-epoxy-3-alpha,7-alpha,15-trihydroxy-9-trichothecen-8-one;4-deoxynivalenol;dehydronivalenol;desoxynivalenol;rdtoxin;Spiro[2,5-methano-1-benzoxepin-10,2'-oxirane], trichothec-9-en-8-one deriv.;Trichothec-9-en-8-one, 12,13-epoxy-3,7,15-trihydroxy-, (3alpha,7alpha)-;DEOXYNIVALENOL,1X1ML, ETOAC/MEOH(95:5),2 00UG/ML;deoxynivalenol solution;TRICHOTHECENEVOMITOXIN;DEOXYNIVALENOL SOLUTION (100ΜG/ML ACETONITORILE SOLUTION);Deoxynivalenol, Vomitoxin;3α,7α,15-Trihydroxy-12,13-epoxytrichothec-9-en-8-one
MF: C15H20O6
FW: 296.32
Melting Point:151-153°C
Flash Point: -3 °C
Storage temp.: 2-8°C
Vomitoxin is a type B trichothecene, an epoxy-sesquiterpeneoid. Vomitoxin occurs predominantly in grains such as wheat, barley, oats, rye, and maize, and less often in rice, sorghum, and triticale.
VOMITOXIN Toxicity Data With Reference
| 1. | skn-gpg 148 μg MLD | FAATDF Fundamental and Applied Toxicology. 4 (2, Pt 2),(1984),S124. | ||
| 2. | orl-mus LD50:46 mg/kg | FAATDF Fundamental and Applied Toxicology. 4 (2, Pt 2),(1984),S124. | ||
| 3. | ipr-mus LD50:43 mg/kg | TOXIA6 Toxicon. 24 (1986),985. | ||
| 4. | scu-mus LD50:45 mg/kg | TOXIA6 Toxicon. 24 (1986),985. | ||
| 5. | scu-dog LD50:27 mg/kg | VHTODE Veterinary and Human Toxicology. 25 (1983),335. |
VOMITOXIN Safety Profile
Poison by ingestion, subcutaneous, and intraperitoneal routes. An experimental teratogen. Experimental reproductive effects. A skin irritant. When heated to decomposition it emits acrid smoke and fumes.
Human: 1 ppm restriction of vomitoxin.
Companion animals: 5 ppm restriction of vomitoxin.The grains is not exceed 40% of the diet.
Livestock and farm animals: 10 ppm restriction of vomitoxin.Ingredients may not exceed 50% of the diet.
Dairy cow :2 ppm restriction of vomitoxin
VOMITOXIN Specification
vomitoxin appears to occur through the central nervous system. Vomitoxin is strong protein inhibitors. vomitoxin causes the brain to increase its uptake of the amino acid tryptophan and, in turn, its synthesis of serotonin. Increased levels of serotonin are believed to be responsible for the anorexic effects of DON and other tricothecenes. Irritation of the gastrointestinal tract may also play a role in reducing feed intake... This fact may also partially explain the high incidence of pars esaughageal stomach ulcers observed in sows off feed during feed refusal.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View