Simagchem Corporation
Welcome to Simagchem, your partner in China as a premier supply of bulk specialty chemicals for industry and life science. We introduce experienced quality product and exceptional JIT service with instant market intelligence in China to benefit our
Cas:105-95-3
Min.Order:1 Kilogram
Negotiable
Type:Manufacturers
inquiryJinan Finer Chemical Co., Ltd
Product description: Product name Ethylene brassylate CAS number 105-95-3 Assay ≥99% Appearance Colorless transparent liquid Capacity 1000mt/year Application Perfume, essenc
Cas:105-95-3
Min.Order:1 Kilogram
FOB Price: $12.0
Type:Lab/Research institutions
inquiryWuhan Fortuna Chemical Co.,Ltd
Hot selling Ethylene brassylate CAS 105-95-3 with competitive price Company profile Wuhan Fortuna Chemical Co.,Ltd established in 2006, is a big integrative chemical enterprise being engaged in Pharmaceutical & its intermediates, Food/Feed a
Cas:105-95-3
Min.Order:10 Kilogram
FOB Price: $10.0
Type:Trading Company
inquiryAlity Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Xi'an Xszo Chem Co., Ltd.
1. Factory price and high quality must be guaranteed, base on 8 years of production and R&D experience2. Free samples will be provided,ensure specifications and quality are right for customer3. Customers will receive the most professional technical s
Cas:105-95-3
Min.Order:1 Gram
FOB Price: $0.1
Type:Manufacturers
inquiryShanghai Seasonsgreen Chemical Co.,Ltd
Shanghai Seasonsgreen Chemical is a high-tech research and development, production, sale and custom synthesis set in one high-tech chemical products enterprises. Our sales and marketing division is located in Shanghai, serving international pharmaceu
Cas:105-95-3
Min.Order:1 Kilogram
FOB Price: $1.0
Type:Manufacturers
inquiryDayang Chem (Hangzhou) Co.,Ltd.
As a leading manufacturer and supplier of chemicals in China, DayangChem not only supply popular chemicals, but also DayangChem’s R&D center offer custom synthesis services. DayangChem can provide different quantities of custom synthesis
Cas:105-95-3
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryChemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Cas:105-95-3
Min.Order:5 Kiloliter
FOB Price: $1.2 / 5.0
Type:Manufacturers
inquiryHenan Tianfu Chemical Co., Ltd.
Unique advantages for Ethylene Brassylate CAS:105-95-3 Guaranteed purity High quality & competitive price Quality control Fast feedback Prompt shipment one of the biggest factory in the world Tianfu Chemical exported this product to ma
Cas:105-95-3
Min.Order:1 Kilogram
FOB Price: $1.0 / 100.0
Type:Lab/Research institutions
inquiryHebei Nengqian Chemical Import and Export Co., LTD
Our advantages: 1. All inquiries will be replied within 12 hours. 2. Dedication to quality, supply & service. 3. Strictly on selecting raw materials. 4. Reasonable & competitive price, fast lead time. 5. Sample is available for your eva
Cas:105-95-3
Min.Order:1 Kilogram
FOB Price: $9.0 / 99.0
Type:Trading Company
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:105-95-3
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryHebei yanxi chemical co.,LTD.
hebei yanxi chemical co., LTD who registered capital of 10 million yuan, nearly to $2 million, we have a pharmaceutical raw materials factory production of pharmaceutical raw materials, and a reagent r&d center, and we do research and developmen
Cas:105-95-3
Min.Order:1 Kilogram
FOB Price: $2.0 / 6.0
Type:Trading Company
inquiryHenan Sinotech Import&Export Corporation
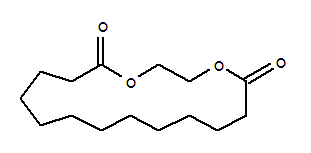
Ethylene brassylate Basic information Product Name: Ethylene brassylate CAS: 105-95-3 MF: C15H26O4 MW: 270.36 EINECS: 203-347-8 Mol File: 105-95-3.mol Appearance:
Hangzhou Sartort Biopharma Co., Ltd
Appearance:Colorless to Light yellow????????clear liquid Storage:room temperature Package:200kg/Drum Application:Chemicals Transportation:Express/Sea/Air Port:Any port in China
Cas:105-95-3
Min.Order:1 Kilogram
FOB Price: $1.0
Type:Lab/Research institutions
inquiryShanghai Upbio Tech Co.,Ltd
1.No Less 8 years exporting experience. Clients can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specialized
Cas:105-95-3
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryQingdao Beluga Import and Export Co., LTD
Ethylene brassylate CAS:105-95-3 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organic interme
Cas:105-95-3
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:105-95-3
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Hangzhou ZeErRui Chemical Co., Ltd.
Product Name Ethylene brassylate CAS No 105-95-3 Structural formula Molecular formula
Cas:105-95-3
Min.Order:100 Gram
Negotiable
Type:Lab/Research institutions
inquiryHangzhou Keyingchem Co.,Ltd
Hangzhou KeyingChem Co., Ltd. exported this product to many countries and regions at best price. If you are looking for the material’s manufacturer or supplier in China, KeyingChem is your best choice. Pls contact with us freely for getting det
Cas:105-95-3
Min.Order:0 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Hangzhou Lingrui Chemical Co.,Ltd.
our strengths: 1: Fast and guaranteed shipment (TNT;EMS;FEDEX;DHL;UPS;EUB, special line) 2: Various payment items accepted (Btc; MoneyGram; WU) 3: Valued package (Paraffin coating; Double aluminum foil bag; Vacuum packaging) 4: Efficient delivery
Cas:105-95-3
Min.Order:1 Gram
Negotiable
Type:Other
inquiryZibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:105-95-3
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryHANGZHOU YUNUO CHEMICAL CO.,LTD
superior quality Appearance:colourless liquid Storage:Stored in cool, dry and ventilation place; Away from fire and heat Package:1kg/bag, 1kg/drum or 25kg/drum or as per your request. Application:Consumption of spices. Used in the vanilla, cinnamon
Siwei Development Group Ltd.
Product name: Ethylene Brassylate CAS No.:105-95-3 Molecule Formula:C15H26O4 Molecule Weight:270.36 Purity: 99% Package: 200kg/drum Description:Colorless or light yellow liquid Manufacture Standards:Enterprise Standard TESTING IT
Cas:105-95-3
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryXiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
Hangzhou Huarong Pharm Co., Ltd.
We Huarong Pharm can provide Customized Synthesis & Process R&D & APIs and intermediates Production & Quality Research & Registration Application, especially our GMP validation service which complies with SFDA, FDA, WHO and EU EMPA. O
Hunan chemfish Pharmaceutical co.,Ltd
Appearance:95%+ Package:R&D,Pilot run Transportation:per client require Port:Express ,Air, Sea
SAGECHEM LIMITED
SAGECHEM is a chemical R&D, manufacturing and distribution company in China since 2009, including pharmaceutical intermediates, agrochemical, dyestuff intermediates, organosilicone, API and etc. We also offer a full range of services in
Cas:105-95-3
Min.Order:0 Metric Ton
Negotiable
Type:Lab/Research institutions
inquirySuzhou Health Chemicals Co., Ltd.
High quality,stable supply chain.Appearance:white/off-white or light yellow Storage:Store in cool and dry place, keep away from strong light and heat. Package:aluminum bottle,glass bottle,PTFE bottle,cardboard drum Application:This product can be use
Synthetic route

| Conditions | Yield |
|---|---|
| With ethylene glycol at 200℃; under 2 Torr; Erhitzen des Reaktionsprodukts mit wenig Zinn(II)-chlorid-dihydrat unter 1 Torr auf 270grad; |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 10h; | 189 mg |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
13362-52-2
1,13-tridecanediol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether for 3.5h; Ambient temperature; | 98% |
| With lithium aluminium tetrahydride In tetrahydrofuran; diethyl ether at 0 - 20℃; Reflux; | 76% |
| With lithium aluminium tetrahydride In diethyl ether Reflux; |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

| Conditions | Yield |
|---|---|
| Candida antarctica lipase at 75℃; for 24h; Polymerization; ring cleavage; |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
116754-58-6
13-bromo-1-tridecylalcohol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: lithium aluminium tetrahydride / tetrahydrofuran; diethyl ether / 0 - 20 °C / Reflux 2: hydrogen bromide / water; toluene / 22 h / Dean-Stark; Reflux View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
50995-26-1
(Z)-14-tricosen-1-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 93 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: H2, quinoline / Lindlar palladium catalyst / hexane / 20.5 h / Ambient temperature View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
62873-30-7
heptadec-16-yn-1-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 99 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: 92 percent / 1,3-propanediamine, Li, potassium tert-butoxide / 2 h / Ambient temperature View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
116452-12-1
2-((13-bromotridecyl)oxy)tetrahydro-2H-pyran

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature View Scheme | |
| Multi-step reaction with 3 steps 1: lithium aluminium tetrahydride / diethyl ether / Reflux 2: hydrogen bromide / toluene / Reflux; Dean-Stark 3: pyridinium p-toluenesulfonate / dichloromethane / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: lithium aluminium tetrahydride / tetrahydrofuran; diethyl ether / 0 - 20 °C / Reflux 2: hydrogen bromide / water; toluene / 22 h / Dean-Stark; Reflux 3: pyridinium p-toluenesulfonate / dichloromethane / 18 h / 20 °C View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
159627-68-6
14-heptadecyn-1-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 99 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
159627-64-2
14-tricosyn-1-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 93 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
159627-66-4
(Z)-14-tricosenyl bromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 93 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: H2, quinoline / Lindlar palladium catalyst / hexane / 20.5 h / Ambient temperature 8: 98 percent / triethylamine, (4-dimethylamino)pyridine / CH2Cl2 / 6 h / Ambient temperature 9: 97 percent / LiBr, NaHCO3 / acetone / 15 h / Ambient temperature View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
159627-62-0
1-tetrahydropyranyloxy-14-pentadecyne

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
159627-71-1
16-tetracosyn-1-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 99 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: 92 percent / 1,3-propanediamine, Li, potassium tert-butoxide / 2 h / Ambient temperature 8: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 9: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 10: 94 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
159627-72-2
(Z)-16-tetracosen-1-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 99 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: 92 percent / 1,3-propanediamine, Li, potassium tert-butoxide / 2 h / Ambient temperature 8: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 9: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 10: 94 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 11: 93 percent / H2, quinoline / Lindlar palladium catalyst / hexane / 20.5 h / Ambient temperature View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
159627-74-4
(Z)-16-tetracosenyl bromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 99 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: 92 percent / 1,3-propanediamine, Li, potassium tert-butoxide / 2 h / Ambient temperature 8: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 9: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 10: 94 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 11: 93 percent / H2, quinoline / Lindlar palladium catalyst / hexane / 20.5 h / Ambient temperature 12: 95 percent / Et3N, DMAP / CH2Cl2 / 6 h / Ambient temperature 13: 97 percent / LiBr, NaHCO3 / acetone / 15 h / Ambient temperature View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
159627-69-7
2-(heptadec-16-yn-1-yloxy)tetrahydro-2H-pyran

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 99 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: 92 percent / 1,3-propanediamine, Li, potassium tert-butoxide / 2 h / Ambient temperature 8: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
159627-67-5
1-tetrahydropyranyloxy-14-heptadecyne

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
159627-82-4
(2S,3R,18Z)-2,3-epoxy-18-heptacosen-1-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 93 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: H2, quinoline / Lindlar palladium catalyst / hexane / 20.5 h / Ambient temperature 8: 98 percent / triethylamine, (4-dimethylamino)pyridine / CH2Cl2 / 6 h / Ambient temperature 9: 97 percent / LiBr, NaHCO3 / acetone / 15 h / Ambient temperature 10: 1.) Mg, 2.) CuI / 1.) ether, r.t., 2 h, 2.) ether, HMPA, r.t., 18 h 11: 95 percent / 1M tetrabutylammonium fluoride / tetrahydrofuran / 0.75 h / 0 °C View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
159627-77-7
(2S,3R,20Z)-2,3-epoxy-20-octacosen-1-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 15 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 99 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: 92 percent / 1,3-propanediamine, Li, potassium tert-butoxide / 2 h / Ambient temperature 8: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 9: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 10: 94 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 11: 93 percent / H2, quinoline / Lindlar palladium catalyst / hexane / 20.5 h / Ambient temperature 12: 95 percent / Et3N, DMAP / CH2Cl2 / 6 h / Ambient temperature 13: 97 percent / LiBr, NaHCO3 / acetone / 15 h / Ambient temperature 14: 1.) Mg, 2.) CuI / 1.) ether, r.t., 2 h, 2.) ether, HMPA, r.t., 18 h 15: 94 percent / 1M tetrabutylammonium fluoride / tetrahydrofuran / 0.75 h / 0 °C View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
159627-63-1
1-tetrahydropyranyloxy-14-tricosyne

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
159627-70-0
1-tetrahydropyranyloxy-16-tetracosyne

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 99 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: 92 percent / 1,3-propanediamine, Li, potassium tert-butoxide / 2 h / Ambient temperature 8: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 9: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
159627-65-3
(Z)-14-tricosenyl tosylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 93 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: H2, quinoline / Lindlar palladium catalyst / hexane / 20.5 h / Ambient temperature 8: 98 percent / triethylamine, (4-dimethylamino)pyridine / CH2Cl2 / 6 h / Ambient temperature View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
159627-73-3
(Z)-16-tetracosenyl tosylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 99 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: 92 percent / 1,3-propanediamine, Li, potassium tert-butoxide / 2 h / Ambient temperature 8: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 9: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 10: 94 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 11: 93 percent / H2, quinoline / Lindlar palladium catalyst / hexane / 20.5 h / Ambient temperature 12: 95 percent / Et3N, DMAP / CH2Cl2 / 6 h / Ambient temperature View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 93 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: H2, quinoline / Lindlar palladium catalyst / hexane / 20.5 h / Ambient temperature 8: 98 percent / triethylamine, (4-dimethylamino)pyridine / CH2Cl2 / 6 h / Ambient temperature 9: 97 percent / LiBr, NaHCO3 / acetone / 15 h / Ambient temperature 10: 1.) Mg, 2.) CuI / 1.) ether, r.t., 2 h, 2.) ether, HMPA, r.t., 18 h 11: 95 percent / 1M tetrabutylammonium fluoride / tetrahydrofuran / 0.75 h / 0 °C 12: 99 percent / triethylamine, (4-dimethylamino)pyridine / CH2Cl2 / 1.5 h / Ambient temperature View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 16 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 99 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: 92 percent / 1,3-propanediamine, Li, potassium tert-butoxide / 2 h / Ambient temperature 8: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 9: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 10: 94 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 11: 93 percent / H2, quinoline / Lindlar palladium catalyst / hexane / 20.5 h / Ambient temperature 12: 95 percent / Et3N, DMAP / CH2Cl2 / 6 h / Ambient temperature 13: 97 percent / LiBr, NaHCO3 / acetone / 15 h / Ambient temperature 14: 1.) Mg, 2.) CuI / 1.) ether, r.t., 2 h, 2.) ether, HMPA, r.t., 18 h 15: 94 percent / 1M tetrabutylammonium fluoride / tetrahydrofuran / 0.75 h / 0 °C 16: 99 percent / triethylamine, (4-dimethylamino)pyridine / CH2Cl2 / 1.5 h / Ambient temperature View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
159627-81-3
(2S,3R,18Z)-1-tert-butyldiphenylsilyloxy-2,3-epoxy-18-heptacosene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 93 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: H2, quinoline / Lindlar palladium catalyst / hexane / 20.5 h / Ambient temperature 8: 98 percent / triethylamine, (4-dimethylamino)pyridine / CH2Cl2 / 6 h / Ambient temperature 9: 97 percent / LiBr, NaHCO3 / acetone / 15 h / Ambient temperature 10: 1.) Mg, 2.) CuI / 1.) ether, r.t., 2 h, 2.) ether, HMPA, r.t., 18 h View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

-
-
159627-76-6
(2S,3R,20Z)-1-tert-butyldiphenylsilyloxy-2,3-epoxy-20-octacosene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 99 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: 92 percent / 1,3-propanediamine, Li, potassium tert-butoxide / 2 h / Ambient temperature 8: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 9: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 10: 94 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 11: 93 percent / H2, quinoline / Lindlar palladium catalyst / hexane / 20.5 h / Ambient temperature 12: 95 percent / Et3N, DMAP / CH2Cl2 / 6 h / Ambient temperature 13: 97 percent / LiBr, NaHCO3 / acetone / 15 h / Ambient temperature 14: 1.) Mg, 2.) CuI / 1.) ether, r.t., 2 h, 2.) ether, HMPA, r.t., 18 h View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 93 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: H2, quinoline / Lindlar palladium catalyst / hexane / 20.5 h / Ambient temperature View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 99 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: 92 percent / 1,3-propanediamine, Li, potassium tert-butoxide / 2 h / Ambient temperature 8: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 9: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 10: 94 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 11: 93 percent / H2, quinoline / Lindlar palladium catalyst / hexane / 20.5 h / Ambient temperature 12: 95 percent / Et3N, DMAP / CH2Cl2 / 6 h / Ambient temperature 13: 97 percent / LiBr, NaHCO3 / acetone / 15 h / Ambient temperature 14: 1.) Mg, 2.) CuI / 1.) ether, r.t., 2.5 h, 2.) ether, HMPA, r.t., 6 h View Scheme | |
| Multi-step reaction with 13 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 93 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: H2, quinoline / Lindlar palladium catalyst / hexane / 20.5 h / Ambient temperature 8: 98 percent / triethylamine, (4-dimethylamino)pyridine / CH2Cl2 / 6 h / Ambient temperature 9: 97 percent / LiBr, NaHCO3 / acetone / 15 h / Ambient temperature 10: 1.) Mg, 2.) CuI / 1.) ether, r.t., 2 h, 2.) ether, HMPA, r.t., 18 h 11: 95 percent / 1M tetrabutylammonium fluoride / tetrahydrofuran / 0.75 h / 0 °C 12: 99 percent / triethylamine, (4-dimethylamino)pyridine / CH2Cl2 / 1.5 h / Ambient temperature 13: 1.) Mg, 2.) CuI / 1.) ether, r.t., 2.5 h, 2.) ether, HMPA, r.t., 6 h View Scheme |
-
-
105-95-3
1,4-dioxa-cycloheptadecane-5,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 17 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 99 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: 92 percent / 1,3-propanediamine, Li, potassium tert-butoxide / 2 h / Ambient temperature 8: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 9: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 10: 94 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 11: 93 percent / H2, quinoline / Lindlar palladium catalyst / hexane / 20.5 h / Ambient temperature 12: 95 percent / Et3N, DMAP / CH2Cl2 / 6 h / Ambient temperature 13: 97 percent / LiBr, NaHCO3 / acetone / 15 h / Ambient temperature 14: 1.) Mg, 2.) CuI / 1.) ether, r.t., 2 h, 2.) ether, HMPA, r.t., 18 h 15: 94 percent / 1M tetrabutylammonium fluoride / tetrahydrofuran / 0.75 h / 0 °C 16: 99 percent / triethylamine, (4-dimethylamino)pyridine / CH2Cl2 / 1.5 h / Ambient temperature 17: 1.) Mg, 2.) CuI / 1.) ether, r.t., 2.5 h, 2.) ether, HMPA, r.t., 6 h View Scheme | |
| Multi-step reaction with 10 steps 1: 98 percent / LiAlH4 / diethyl ether / 3.5 h / Ambient temperature 2: 72 percent / 48percent HBr / benzene / 36 h / Heating 3: 98 percent / pyridinium p-toluenesulfonate / CH2Cl2 / 4 h / Ambient temperature 4: 91 percent / xylene; tetrahydrofuran; hexamethylphosphoric acid triamide / 36 h / Ambient temperature 5: 1.) 1.63N butyllithium / 1.) hexane, THF, -50 deg C, 30 min; 0 deg C, 30 min, 2.) hexane, THF, HMPA, -50 deg C, 30 min; 0 deg C, 2 h 6: 93 percent / p-toluenesulfonic acid monohydrate / methanol / 2 h / Ambient temperature 7: H2, quinoline / Lindlar palladium catalyst / hexane / 20.5 h / Ambient temperature 8: 98 percent / triethylamine, (4-dimethylamino)pyridine / CH2Cl2 / 6 h / Ambient temperature 9: 97 percent / LiBr, NaHCO3 / acetone / 15 h / Ambient temperature 10: 1.) Mg, 2.) CuI / 1.) ether, r.t., 2.5 h, 2.) ether, HMPA, r.t., 6 h View Scheme |
Related products
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View






 Xi
Xi