Nantong Noatic Bio-Pharm Technology Co.,Ltd
We are manufacturer of this product and can supply good quality and competitive price , the purity over 99.0%, this product has been in massive production for years , so its process and quality always keep stable and its price is very competitive .
Cas:110351-94-5
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryDayang Chem (Hangzhou) Co.,Ltd.
DayangChem exported this product to many countries and regions at best price. If you are looking for the material's manufacturer or supplier in China, DayangChem is your best choice. Pls contact with us freely for getting detailed product spe
Cas:110351-94-5
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryLIDE PHARMACEUTICALS LIMITED
Advantage : LIDE PHARMACEUTICALS LTD. is a mid-small manufacturing-type enterprise, engaged in pharmaceutical intermediates of R&D, custom-made and production, and also involving trading chemicals for export. We have established the R&D b
Cas:110351-94-5
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by email in time prod
Cas:110351-94-5
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:110351-94-5
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryWuhan Han Sheng New Material Technology Co.,Ltd
Our Advantage: high quality with competitive price High quality standard: BP/USP/EP Enterprise standard All purity customized Fast and safe delivery We have reliable forwarder who can help us deliver our goods more fast and safe. We
Cas:110351-94-5
Min.Order:10 Kilogram
Negotiable
Type:Trading Company
inquiryQingdao Beluga Import and Export Co., LTD
(S)-4-Ethyl-4-hydroxy-7,8-dihydro-1h-pyrano[3,4-f]indolizine-3,6,10(4h)-trione CAS:110351-94-5 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemic
Cas:110351-94-5
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:110351-94-5
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryBaoji Guokang Healthchem co.,ltd
Our company has been in existence for 10 years since its establishment. We have our own unique team. The company integrates independent research and development, production and sales. We have established famous brands at home and abroad. At present
Cas:110351-94-5
Min.Order:1 Gram
FOB Price: $1035.0
Type:Trading Company
inquiryAfine Chemicals Limited
Company Introduction 1. Established in 2005, with two independent business divisions: Fine chemicals division; Pharmaceutical division. 2. Main product: Optical brightener Textile auxiliary Dye stuff Pigments
Cas:110351-94-5
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquirySHANGHAI T&W PHARMACEUTICAL CO., LTD.
A substitute for perfluorooctanoic acid, mainly used as a surfactant, dispersant, additive, etc Appearance:White solid or Colorless liquid Purity:99.3 % We will ship the goods in a timely manner as required We can provide relevant documents acc
Cas:110351-94-5
Min.Order:4 Kilogram
Negotiable
Type:Lab/Research institutions
inquirySaiwante (shanghai) New Material Technology Co., Ltd.
Melting Point:183-185℃ (decomposition) Boiling Point:666.634 °C at 760 mmHg PKA:11.20±0.20(Predicted) Flash Point:356.968 °C PSA:85.60000 Density:1.506 g/cm3 LogP:0.08910 Storage Temp.:2-8°C XLogP3:-0.8 Hydrogen
Cas:110351-94-5
Min.Order:1 Gram
FOB Price: $3.0
Type:Trading Company
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Cas:110351-94-5
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryZibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:110351-94-5
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquirySHANGHAI SYSTEAM BIOCHEM CO., LTD
We are one of a few suppliers that can offer custom synthesis service of this product We are specialized in custom synthesis, chemical/pharmaceutical/ pesticides outsourcing and contract research. We are committed to prov
Cas:110351-94-5
Min.Order:100 Gram
FOB Price: $100.0 / 2000.0
Type:Lab/Research institutions
inquirySiwei Development Group Ltd.
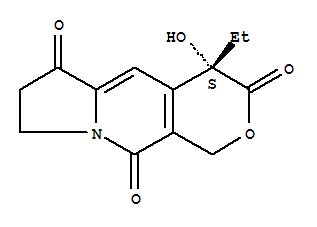
Product name: (S)-4-Ethyl-4-Hydroxy-7,8-Dihydro-1H-Pyrano[3,4-f]Indolizine-3,6,10(4H)-Trione CAS No.:110351-94-5 Molecule Formula:C13H13NO5 Molecule Weight:263.64 Purity: 99% Package: 25kg/drum Description:White crystalline powder Manufacture
Cas:110351-94-5
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryZibo Dorne chemical technology co. LTD
Product Details Grade: pharmaceutical grade Purity:99%+ ProductionCapacity: 1000 Kilogram/Month Scope of use: For scientific research only(The product must be used legally) Our Advantage 1. Best quality with competitive price. 2. Quick shipping,
Cas:110351-94-5
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryXiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
Cas:110351-94-5
Min.Order:1 Milligram
Negotiable
Type:Trading Company
inquiryEAST CHEMSOURCES LIMITED
factory?direct?saleAppearance:White Powder Storage:Store In Dry, Cool And Ventilated Place Package:25kg/drum, also according to the clients requirement Application:It is widely used as a thickener, emulsifier and stabilizer Transportation:By Sea Or B
Cas:110351-94-5
Min.Order:1 Kilogram
FOB Price: $18.0 / 20.0
Type:Trading Company
inquiryHangzhou Huarong Pharm Co., Ltd.
We Huarong Pharm can provide Customized Synthesis & Process R&D & APIs and intermediates Production & Quality Research & Registration Application, especially our GMP validation service which complies with SFDA, FDA, WHO and EU EMPA. O
Cas:110351-94-5
Min.Order:0
Negotiable
Type:Trading Company
inquiryShandong Mopai Biotechnology Co., LTD
Shandong Mopai Biotechnology Co., LTD is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemicals. W
Cas:110351-94-5
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryKAISA GROUP INC
1.Applied in food field.it can improve the immune system and prolong life. 2.Appliedin cosmetic field.it can improve the skin care. 3.Applied in pharmaceutical field.it can treat various dieases. 4.Our product quality assurance will make our customer
Cas:110351-94-5
Min.Order:1 Metric Ton
FOB Price: $1.5
Type:Trading Company
inquirySuzhou Health Chemicals Co., Ltd.
High quality,stable supply chain.Appearance:white/off-white or light yellow Storage:Store in cool and dry place, keep away from strong light and heat. Package:aluminum bottle,glass bottle,PTFE bottle,cardboard drum Application:This product can be use
Cas:110351-94-5
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryChemvon Biotechnology Co. Ltd.
Chemvon Biotechnology Co. Ltd., established in the end of 2004, is a high-technology pharmaceutical and fine chemical company in R&D, manufacturing and sales of (S)-4-ETHYL-4-HYDROXY-7,8-DIHYDRO-1H-PYRANO[3,4-F]INDOLIZINE-3,6,10(4H)-TRIONE. With
Cas:110351-94-5
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryBluecrystal chem-union
We are a Union of chemistry in China, consists of chemists,engineers, laboratories,factories in China. We organize surplus capacity of R&D and production as well as custom synthesis for chemical products and chemical business project. We are supp
Cas:110351-94-5
Min.Order:1 Metric Ton
FOB Price: $1.0
Type:Trading Company
inquiryWin-Win chemical Co.Ltd
Stock products, own laboratory Package:Grams, Kilograms Application:For R&D Transportation:According to customer request Port:Shanghai
Cas:110351-94-5
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquirySuzhou Sibian Chemical Technology Co., Ltd
The process is mature and can be mass produced to 100 kg, with good quality and purity up to 99%.The product has a large stock and can be supplied stably. Package:bottle Application:Drug R&D Transportation:Sealed drying(25℃) Port:ShangHai
Cas:110351-94-5
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryGIHI CHEMICALS CO.,LIMITED
Lower price, sample is available,SDS test documents are available,large stock in warehouseAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:Fine chemical intermediates, used as the main raw material for the synthe
Cas:110351-94-5
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHangzhou Dingyan Chem Co., Ltd
R & D enterprises have their own stock in stock Package:1kg Application:pharmaceutical intermediates
Cas:110351-94-5
Min.Order:0
Negotiable
Type:Manufacturers
inquiryXi`an Eastling Biotech Co., Ltd.
high purity lowest priceAppearance:solid or liquid Storage:in sealed air resistant place Package:Foil bag; Drum; Plastic bottle Application:Pharma;Industry;Agricultural Transportation:by sea or air Port:Beijing or Guangzhou
Cas:110351-94-5
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquirySynthetic route
-
-
110351-93-4
(S)-4′-ethyl-4′-hydroxy-7′,8′-dihydrospiro[[1,3]dioxolane-2,6′-pyrano[3,4-f]indolizine]-3′,10′(1′H,4′H)-dione

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran; water at 60℃; for 3h; | 100% |
| With trifluoroacetic acid at 20℃; for 6h; | 93% |
| With trifluoroacetic acid for 3h; Ambient temperature; | 88.9% |
-
-
165674-36-2
C17H21NO7

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran; water at 60℃; for 3h; | 100% |

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Stage #1: (S,E)-6-benzylidene-4-ethyl-4-hydroxy-7,8-dihydro-1H-pyrano[3,4-f]indolizine-3,10(4H,6H)-dione With oxygen; ozone In dichloromethane at -40℃; for 0.75h; Stage #2: With dimethylsulfide In dichloromethane at -40 - 20℃; for 2h; | 96% |
-
-
110351-93-4
(S)-4′-ethyl-4′-hydroxy-7′,8′-dihydrospiro[[1,3]dioxolane-2,6′-pyrano[3,4-f]indolizine]-3′,10′(1′H,4′H)-dione

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran; water at 60℃; for 3h; | 100% |
| With trifluoroacetic acid at 20℃; for 6h; | 93% |
| With trifluoroacetic acid for 3h; Ambient temperature; | 88.9% |
-
-
165674-36-2
C17H21NO7

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran; water at 60℃; for 3h; | 100% |

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Stage #1: (S,E)-6-benzylidene-4-ethyl-4-hydroxy-7,8-dihydro-1H-pyrano[3,4-f]indolizine-3,10(4H,6H)-dione With oxygen; ozone In dichloromethane at -40℃; for 0.75h; Stage #2: With dimethylsulfide In dichloromethane at -40 - 20℃; for 2h; | 96% |
-
-
183434-04-0
1,1-dimethylethyl (S)-4-ethyl-3,4,8,10-tetrahydro-4,6-dihydroxy-3,10-dioxo-1H-pyrano[3,4 - f] indolidin-7-carboxylate

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| In toluene; trifluoroacetic acid at 110℃; for 2h; | 93.4% |
| With trifluoroacetic acid In toluene at 110℃; for 1.66667h; | 77% |
-
-
110314-10-8
(S)-α-ethyl-α-hydroxy-1,1-(ethylenedioxy)-6-hydroxymethyl-5-oxo-1,2,3,5-tetrahydroindolizine-7-acetamide

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid for 3h; Ambient temperature; | 93% |
| With sulfuric acid In 1,2-dimethoxyethane | |
| Multi-step reaction with 2 steps 1: acetic acid / 2 h / 70 °C 2: 2N aq. sulfuric acid / 1,2-dimethoxy-ethane / 8 h / 50 °C View Scheme |
-
-
102978-41-6
4'-ethyl-1',4',7',8'-tetrahydro-4'-hydroxy-3'H,10'H-spiro[1,3-dioxolane-2,6'-pyrano[3,4-f]indolizine]-3',10'-dione

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 98.1 percent / DMAP / CH2Cl2 / 4 h / 40 °C 2: aq. K2CO3 / methanol / Ambient temperature 3: 88.9 percent / 80percent aq. CF3COOH / 3 h / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 1: 20 h / 75 °C 2: sulfuric acid / 1,2-dimethoxy-ethane View Scheme | |
| Multi-step reaction with 3 steps 1: 20 h / 75 °C 2: acetic acid / 2 h / 70 °C 3: 2N aq. sulfuric acid / 1,2-dimethoxy-ethane / 8 h / 50 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 20 h / 70 °C 2: p-toluenesulfonica acid / acetic acid / 7 h / 70 °C 3: 2N aq. sulfuric acid / 1,2-dimethoxy-ethane / 8 h / 50 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: copper(l) chloride / dichloromethane / 8 h / 20 °C 2.1: potassium hydroxide / methanol / 5 h / 67 °C 2.2: pH 2-3 3.1: trifluoroacetic acid / 6 h / 20 °C View Scheme |
-
-
127318-97-2
1,1-ethylenedioxy-5-oxo-(5'-ethyl-2'H,5'H,6'H-6-oxopyran)-[3',4',f]-Δ6(8)-tetrahydroindolizine

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: O2, (EtO)3P, tBuONa / dimethylformamide / 2 h / -40 °C 2: 98.1 percent / DMAP / CH2Cl2 / 4 h / 40 °C 3: aq. K2CO3 / methanol / Ambient temperature 4: 88.9 percent / 80percent aq. CF3COOH / 3 h / Ambient temperature View Scheme | |
| Multi-step reaction with 8 steps 1: 94 percent / DIBAL-H / tetrahydrofuran / 2 h / -78 °C 2: 96 percent / MsCl, TEA / tetrahydrofuran / 24 h / Ambient temperature 3: (DHQD)2-PHAL, K3Fe(CN)6, K2CO3, K2OsO2, CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 96 h / Ambient temperature 4: pyridine / 3 h / Ambient temperature 5: 93 percent / i-PrNEt / CH2Cl2 / 3 h / Ambient temperature 6: 92 percent / aq. K2CO3 / methanol / 2 h / Ambient temperature 7: 84 percent / pyridinium chlorochromate, AcONa, molecular sieves 4 Angstroem / 24 h / Ambient temperature 8: 100 percent / aq. HCl / tetrahydrofuran; H2O / 3 h / 60 °C View Scheme | |
| Multi-step reaction with 5 steps 1: 94 percent / DIBAL-H / tetrahydrofuran / 2 h / -78 °C 2: 96 percent / MsCl, TEA / tetrahydrofuran / 24 h / Ambient temperature 3: (DHQD)2-PHAL, K3Fe(CN)6, K2CO3, K2OsO2, CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 96 h / Ambient temperature 4: I2, CaCO3 / methanol; H2O / 24 h / Ambient temperature 5: 100 percent / aq. HCl / tetrahydrofuran; H2O / 3 h / 60 °C View Scheme |
-
-
144788-94-3
4-acetoxy-4-ethyl-6,6-(ethylenedioxy)-7,8-dihydro-1H-pyrano[3,4-f]indolizine-3,10(4H)-dione

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. K2CO3 / methanol / Ambient temperature 2: 88.9 percent / 80percent aq. CF3COOH / 3 h / Ambient temperature View Scheme |
-
-
99-11-6
citrazinic acid

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 17 steps 1: 78 percent / POPCl3, Me4NCl / 2 h / 130 - 142 °C 2: 84 percent / tetrahydrofuran / 1 h / 0 °C 3: 100 percent / TMSCl / 12 h / Ambient temperature 4: 89 percent / methanol / 20 h / Heating 5: 1.) n-BuLi / 1.) heptane, hexane, 0 deg C, 30 min, 2.) hexane, heptane, -30 deg C - 0 deg C, 1 h 6: 71 percent / NaBH4, n-Bu4NCl / H2O / 18 h 7: 99.3 percent / t-BuOK / tetrahydrofuran / 1 h / 20 - 30 °C 8: 89 percent / Pd(OAc)2, K2CO3, DPP / dimethylformamide / 16 h / 90 °C / 775.7 Torr 10: 92 percent / OsO4, Me3NO*2H2O / 2-methyl-propan-2-ol / 24 h / 40 °C 11: 76 percent / PS-30 catalyst / various solvent(s) / 48 h 12: 100 percent / NaOCl, 4-acetoxy-TEMPO, KBr, NaHCO3 / CH2Cl2; H2O / 0.67 h 13: 96 percent / H2 / Pd/C / methanol / 96 h / 775.7 Torr / Ambient temperature; other catalyst, reaction time, temperature 14: 99 percent / TEMPO, KBr, NaHCO3, NaOCl / CH2Cl2; H2O / 0.5 h 15: 89 percent / TMSCl, NaI / acetonitrile / 6 h / 0 - 20 °C 16: 74 percent / Cs2CO3 / dimethylsulfoxide / 21 h / 47 - 50 °C 17: 93.4 percent / trifluoroacetic acid; toluene / 2 h / 110 °C View Scheme |
-
-
5398-44-7
2,6-dichloropyridine-4-carboxylic acid

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 16 steps 1: 84 percent / tetrahydrofuran / 1 h / 0 °C 2: 100 percent / TMSCl / 12 h / Ambient temperature 3: 89 percent / methanol / 20 h / Heating 4: 1.) n-BuLi / 1.) heptane, hexane, 0 deg C, 30 min, 2.) hexane, heptane, -30 deg C - 0 deg C, 1 h 5: 71 percent / NaBH4, n-Bu4NCl / H2O / 18 h 6: 99.3 percent / t-BuOK / tetrahydrofuran / 1 h / 20 - 30 °C 7: 89 percent / Pd(OAc)2, K2CO3, DPP / dimethylformamide / 16 h / 90 °C / 775.7 Torr 9: 92 percent / OsO4, Me3NO*2H2O / 2-methyl-propan-2-ol / 24 h / 40 °C 10: 76 percent / PS-30 catalyst / various solvent(s) / 48 h 11: 100 percent / NaOCl, 4-acetoxy-TEMPO, KBr, NaHCO3 / CH2Cl2; H2O / 0.67 h 12: 96 percent / H2 / Pd/C / methanol / 96 h / 775.7 Torr / Ambient temperature; other catalyst, reaction time, temperature 13: 99 percent / TEMPO, KBr, NaHCO3, NaOCl / CH2Cl2; H2O / 0.5 h 14: 89 percent / TMSCl, NaI / acetonitrile / 6 h / 0 - 20 °C 15: 74 percent / Cs2CO3 / dimethylsulfoxide / 21 h / 47 - 50 °C 16: 93.4 percent / trifluoroacetic acid; toluene / 2 h / 110 °C View Scheme |
-
-
183433-62-7
1-(2,6-dichloropyridin-4-yl)propan-1-one

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 15 steps 1: 100 percent / TMSCl / 12 h / Ambient temperature 2: 89 percent / methanol / 20 h / Heating 3: 1.) n-BuLi / 1.) heptane, hexane, 0 deg C, 30 min, 2.) hexane, heptane, -30 deg C - 0 deg C, 1 h 4: 71 percent / NaBH4, n-Bu4NCl / H2O / 18 h 5: 99.3 percent / t-BuOK / tetrahydrofuran / 1 h / 20 - 30 °C 6: 89 percent / Pd(OAc)2, K2CO3, DPP / dimethylformamide / 16 h / 90 °C / 775.7 Torr 8: 92 percent / OsO4, Me3NO*2H2O / 2-methyl-propan-2-ol / 24 h / 40 °C 9: 76 percent / PS-30 catalyst / various solvent(s) / 48 h 10: 100 percent / NaOCl, 4-acetoxy-TEMPO, KBr, NaHCO3 / CH2Cl2; H2O / 0.67 h 11: 96 percent / H2 / Pd/C / methanol / 96 h / 775.7 Torr / Ambient temperature; other catalyst, reaction time, temperature 12: 99 percent / TEMPO, KBr, NaHCO3, NaOCl / CH2Cl2; H2O / 0.5 h 13: 89 percent / TMSCl, NaI / acetonitrile / 6 h / 0 - 20 °C 14: 74 percent / Cs2CO3 / dimethylsulfoxide / 21 h / 47 - 50 °C 15: 93.4 percent / trifluoroacetic acid; toluene / 2 h / 110 °C View Scheme |
-
-
183433-74-1
5-Benzyloxymethyl-4-(1-hydroxy-1-hydroxymethyl-propyl)-6-methoxy-pyridine-2-carboxylic acid propyl ester

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 76 percent / PS-30 catalyst / various solvent(s) / 48 h 2: 100 percent / NaOCl, 4-acetoxy-TEMPO, KBr, NaHCO3 / CH2Cl2; H2O / 0.67 h 3: 96 percent / H2 / Pd/C / methanol / 96 h / 775.7 Torr / Ambient temperature; other catalyst, reaction time, temperature 4: 99 percent / TEMPO, KBr, NaHCO3, NaOCl / CH2Cl2; H2O / 0.5 h 5: 89 percent / TMSCl, NaI / acetonitrile / 6 h / 0 - 20 °C 6: 74 percent / Cs2CO3 / dimethylsulfoxide / 21 h / 47 - 50 °C 7: 93.4 percent / trifluoroacetic acid; toluene / 2 h / 110 °C View Scheme |
-
-
183433-75-2
5-Benzyloxymethyl-4-((S)-1-hydroxy-1-hydroxymethyl-propyl)-6-methoxy-pyridine-2-carboxylic acid propyl ester

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 100 percent / NaOCl, 4-acetoxy-TEMPO, KBr, NaHCO3 / CH2Cl2; H2O / 0.67 h 2: 96 percent / H2 / Pd/C / methanol / 96 h / 775.7 Torr / Ambient temperature; other catalyst, reaction time, temperature 3: 99 percent / TEMPO, KBr, NaHCO3, NaOCl / CH2Cl2; H2O / 0.5 h 4: 89 percent / TMSCl, NaI / acetonitrile / 6 h / 0 - 20 °C 5: 74 percent / Cs2CO3 / dimethylsulfoxide / 21 h / 47 - 50 °C 6: 93.4 percent / trifluoroacetic acid; toluene / 2 h / 110 °C View Scheme |
-
-
183433-63-8
2,6-dichloro-4-(2-ethyl-1,3-dioxolan-2-yl)pyridine

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1: 89 percent / methanol / 20 h / Heating 2: 1.) n-BuLi / 1.) heptane, hexane, 0 deg C, 30 min, 2.) hexane, heptane, -30 deg C - 0 deg C, 1 h 3: 71 percent / NaBH4, n-Bu4NCl / H2O / 18 h 4: 99.3 percent / t-BuOK / tetrahydrofuran / 1 h / 20 - 30 °C 5: 89 percent / Pd(OAc)2, K2CO3, DPP / dimethylformamide / 16 h / 90 °C / 775.7 Torr 7: 92 percent / OsO4, Me3NO*2H2O / 2-methyl-propan-2-ol / 24 h / 40 °C 8: 76 percent / PS-30 catalyst / various solvent(s) / 48 h 9: 100 percent / NaOCl, 4-acetoxy-TEMPO, KBr, NaHCO3 / CH2Cl2; H2O / 0.67 h 10: 96 percent / H2 / Pd/C / methanol / 96 h / 775.7 Torr / Ambient temperature; other catalyst, reaction time, temperature 11: 99 percent / TEMPO, KBr, NaHCO3, NaOCl / CH2Cl2; H2O / 0.5 h 12: 89 percent / TMSCl, NaI / acetonitrile / 6 h / 0 - 20 °C 13: 74 percent / Cs2CO3 / dimethylsulfoxide / 21 h / 47 - 50 °C 14: 93.4 percent / trifluoroacetic acid; toluene / 2 h / 110 °C View Scheme |
-
-
183433-64-9
6-chloro-4-(2-ethyl-1,3-dioxolan-2-yl)-2-methoxypyridine

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1: 1.) n-BuLi / 1.) heptane, hexane, 0 deg C, 30 min, 2.) hexane, heptane, -30 deg C - 0 deg C, 1 h 2: 71 percent / NaBH4, n-Bu4NCl / H2O / 18 h 3: 99.3 percent / t-BuOK / tetrahydrofuran / 1 h / 20 - 30 °C 4: 89 percent / Pd(OAc)2, K2CO3, DPP / dimethylformamide / 16 h / 90 °C / 775.7 Torr 6: 92 percent / OsO4, Me3NO*2H2O / 2-methyl-propan-2-ol / 24 h / 40 °C 7: 76 percent / PS-30 catalyst / various solvent(s) / 48 h 8: 100 percent / NaOCl, 4-acetoxy-TEMPO, KBr, NaHCO3 / CH2Cl2; H2O / 0.67 h 9: 96 percent / H2 / Pd/C / methanol / 96 h / 775.7 Torr / Ambient temperature; other catalyst, reaction time, temperature 10: 99 percent / TEMPO, KBr, NaHCO3, NaOCl / CH2Cl2; H2O / 0.5 h 11: 89 percent / TMSCl, NaI / acetonitrile / 6 h / 0 - 20 °C 12: 74 percent / Cs2CO3 / dimethylsulfoxide / 21 h / 47 - 50 °C 13: 93.4 percent / trifluoroacetic acid; toluene / 2 h / 110 °C View Scheme |
-
-
183433-65-0
6-Chloro-4-(2-ethyl-[1,3]dioxolan-2-yl)-2-methoxy-pyridine-3-carbaldehyde

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1: 71 percent / NaBH4, n-Bu4NCl / H2O / 18 h 2: 99.3 percent / t-BuOK / tetrahydrofuran / 1 h / 20 - 30 °C 3: 89 percent / Pd(OAc)2, K2CO3, DPP / dimethylformamide / 16 h / 90 °C / 775.7 Torr 5: 92 percent / OsO4, Me3NO*2H2O / 2-methyl-propan-2-ol / 24 h / 40 °C 6: 76 percent / PS-30 catalyst / various solvent(s) / 48 h 7: 100 percent / NaOCl, 4-acetoxy-TEMPO, KBr, NaHCO3 / CH2Cl2; H2O / 0.67 h 8: 96 percent / H2 / Pd/C / methanol / 96 h / 775.7 Torr / Ambient temperature; other catalyst, reaction time, temperature 9: 99 percent / TEMPO, KBr, NaHCO3, NaOCl / CH2Cl2; H2O / 0.5 h 10: 89 percent / TMSCl, NaI / acetonitrile / 6 h / 0 - 20 °C 11: 74 percent / Cs2CO3 / dimethylsulfoxide / 21 h / 47 - 50 °C 12: 93.4 percent / trifluoroacetic acid; toluene / 2 h / 110 °C View Scheme |
-
-
183433-66-1
[6-Chloro-4-(2-ethyl-[1,3]dioxolan-2-yl)-2-methoxy-pyridin-3-yl]-methanol

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1: 99.3 percent / t-BuOK / tetrahydrofuran / 1 h / 20 - 30 °C 2: 89 percent / Pd(OAc)2, K2CO3, DPP / dimethylformamide / 16 h / 90 °C / 775.7 Torr 4: 92 percent / OsO4, Me3NO*2H2O / 2-methyl-propan-2-ol / 24 h / 40 °C 5: 76 percent / PS-30 catalyst / various solvent(s) / 48 h 6: 100 percent / NaOCl, 4-acetoxy-TEMPO, KBr, NaHCO3 / CH2Cl2; H2O / 0.67 h 7: 96 percent / H2 / Pd/C / methanol / 96 h / 775.7 Torr / Ambient temperature; other catalyst, reaction time, temperature 8: 99 percent / TEMPO, KBr, NaHCO3, NaOCl / CH2Cl2; H2O / 0.5 h 9: 89 percent / TMSCl, NaI / acetonitrile / 6 h / 0 - 20 °C 10: 74 percent / Cs2CO3 / dimethylsulfoxide / 21 h / 47 - 50 °C 11: 93.4 percent / trifluoroacetic acid; toluene / 2 h / 110 °C View Scheme |
-
-
194999-55-8
(S)-4-Ethyl-3,4-dihydroxy-8-methoxy-3,4-dihydro-1H-pyrano[3,4-c]pyridine-6-carboxylic acid propyl ester

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 99 percent / TEMPO, KBr, NaHCO3, NaOCl / CH2Cl2; H2O / 0.5 h 2: 89 percent / TMSCl, NaI / acetonitrile / 6 h / 0 - 20 °C 3: 74 percent / Cs2CO3 / dimethylsulfoxide / 21 h / 47 - 50 °C 4: 93.4 percent / trifluoroacetic acid; toluene / 2 h / 110 °C View Scheme |
-
-
183434-02-8
propyl (S)-4-ethyl-3,4,7,8-tetrahydro-4-hydroxy-3,8-dioxo-1H-pyrano[3,4-c] pyridine-6-carboxylate

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 74 percent / Cs2CO3 / dimethylsulfoxide / 21 h / 47 - 50 °C 2: 93.4 percent / trifluoroacetic acid; toluene / 2 h / 110 °C View Scheme |
-
-
183434-00-6
propyl (S)-4-ethyl-3,4-dihydro-4-hydroxy-8-methoxy-3-oxo-1H-pyrano[3,4-c]pyridine-6-carboxylate

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 89 percent / TMSCl, NaI / acetonitrile / 6 h / 0 - 20 °C 2: 74 percent / Cs2CO3 / dimethylsulfoxide / 21 h / 47 - 50 °C 3: 93.4 percent / trifluoroacetic acid; toluene / 2 h / 110 °C View Scheme |
-
-
183433-67-2
3-Benzyloxymethyl-6-chloro-4-(2-ethyl-[1,3]dioxolan-2-yl)-2-methoxy-pyridine

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 89 percent / Pd(OAc)2, K2CO3, DPP / dimethylformamide / 16 h / 90 °C / 775.7 Torr 3: 92 percent / OsO4, Me3NO*2H2O / 2-methyl-propan-2-ol / 24 h / 40 °C 4: 76 percent / PS-30 catalyst / various solvent(s) / 48 h 5: 100 percent / NaOCl, 4-acetoxy-TEMPO, KBr, NaHCO3 / CH2Cl2; H2O / 0.67 h 6: 96 percent / H2 / Pd/C / methanol / 96 h / 775.7 Torr / Ambient temperature; other catalyst, reaction time, temperature 7: 99 percent / TEMPO, KBr, NaHCO3, NaOCl / CH2Cl2; H2O / 0.5 h 8: 89 percent / TMSCl, NaI / acetonitrile / 6 h / 0 - 20 °C 9: 74 percent / Cs2CO3 / dimethylsulfoxide / 21 h / 47 - 50 °C 10: 93.4 percent / trifluoroacetic acid; toluene / 2 h / 110 °C View Scheme |
-
-
183433-72-9
5-Benzyloxymethyl-6-methoxy-4-(1-methylene-propyl)-pyridine-2-carboxylic acid propyl ester

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 92 percent / OsO4, Me3NO*2H2O / 2-methyl-propan-2-ol / 24 h / 40 °C 2: 76 percent / PS-30 catalyst / various solvent(s) / 48 h 3: 100 percent / NaOCl, 4-acetoxy-TEMPO, KBr, NaHCO3 / CH2Cl2; H2O / 0.67 h 4: 96 percent / H2 / Pd/C / methanol / 96 h / 775.7 Torr / Ambient temperature; other catalyst, reaction time, temperature 5: 99 percent / TEMPO, KBr, NaHCO3, NaOCl / CH2Cl2; H2O / 0.5 h 6: 89 percent / TMSCl, NaI / acetonitrile / 6 h / 0 - 20 °C 7: 74 percent / Cs2CO3 / dimethylsulfoxide / 21 h / 47 - 50 °C 8: 93.4 percent / trifluoroacetic acid; toluene / 2 h / 110 °C View Scheme |
-
-
183433-96-7
5-Benzyloxymethyl-4-((S)-1-formyl-1-hydroxy-propyl)-6-methoxy-pyridine-2-carboxylic acid propyl ester

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 96 percent / H2 / Pd/C / methanol / 96 h / 775.7 Torr / Ambient temperature; other catalyst, reaction time, temperature 2: 99 percent / TEMPO, KBr, NaHCO3, NaOCl / CH2Cl2; H2O / 0.5 h 3: 89 percent / TMSCl, NaI / acetonitrile / 6 h / 0 - 20 °C 4: 74 percent / Cs2CO3 / dimethylsulfoxide / 21 h / 47 - 50 °C 5: 93.4 percent / trifluoroacetic acid; toluene / 2 h / 110 °C View Scheme |
-
-
183433-68-3
5-Benzyloxymethyl-4-(2-ethyl-[1,3]dioxolan-2-yl)-6-methoxy-pyridine-2-carboxylic acid propyl ester

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 2: 92 percent / OsO4, Me3NO*2H2O / 2-methyl-propan-2-ol / 24 h / 40 °C 3: 76 percent / PS-30 catalyst / various solvent(s) / 48 h 4: 100 percent / NaOCl, 4-acetoxy-TEMPO, KBr, NaHCO3 / CH2Cl2; H2O / 0.67 h 5: 96 percent / H2 / Pd/C / methanol / 96 h / 775.7 Torr / Ambient temperature; other catalyst, reaction time, temperature 6: 99 percent / TEMPO, KBr, NaHCO3, NaOCl / CH2Cl2; H2O / 0.5 h 7: 89 percent / TMSCl, NaI / acetonitrile / 6 h / 0 - 20 °C 8: 74 percent / Cs2CO3 / dimethylsulfoxide / 21 h / 47 - 50 °C 9: 93.4 percent / trifluoroacetic acid; toluene / 2 h / 110 °C View Scheme |
-
-
165674-31-7
C15H17NO4

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: (DHQD)2-PHAL, K3Fe(CN)6, K2CO3, K2OsO2, CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 96 h / Ambient temperature 2: pyridine / 3 h / Ambient temperature 3: 93 percent / i-PrNEt / CH2Cl2 / 3 h / Ambient temperature 4: 92 percent / aq. K2CO3 / methanol / 2 h / Ambient temperature 5: 84 percent / pyridinium chlorochromate, AcONa, molecular sieves 4 Angstroem / 24 h / Ambient temperature 6: 100 percent / aq. HCl / tetrahydrofuran; H2O / 3 h / 60 °C View Scheme | |
| Multi-step reaction with 3 steps 1: (DHQD)2-PHAL, K3Fe(CN)6, K2CO3, K2OsO2, CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 96 h / Ambient temperature 2: I2, CaCO3 / methanol; H2O / 24 h / Ambient temperature 3: 100 percent / aq. HCl / tetrahydrofuran; H2O / 3 h / 60 °C View Scheme |
-
-
165674-30-6
C15H19NO5

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 96 percent / MsCl, TEA / tetrahydrofuran / 24 h / Ambient temperature 2: (DHQD)2-PHAL, K3Fe(CN)6, K2CO3, K2OsO2, CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 96 h / Ambient temperature 3: pyridine / 3 h / Ambient temperature 4: 93 percent / i-PrNEt / CH2Cl2 / 3 h / Ambient temperature 5: 92 percent / aq. K2CO3 / methanol / 2 h / Ambient temperature 6: 84 percent / pyridinium chlorochromate, AcONa, molecular sieves 4 Angstroem / 24 h / Ambient temperature 7: 100 percent / aq. HCl / tetrahydrofuran; H2O / 3 h / 60 °C View Scheme | |
| Multi-step reaction with 4 steps 1: 96 percent / MsCl, TEA / tetrahydrofuran / 24 h / Ambient temperature 2: (DHQD)2-PHAL, K3Fe(CN)6, K2CO3, K2OsO2, CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 96 h / Ambient temperature 3: I2, CaCO3 / methanol; H2O / 24 h / Ambient temperature 4: 100 percent / aq. HCl / tetrahydrofuran; H2O / 3 h / 60 °C View Scheme |

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 84 percent / pyridinium chlorochromate, AcONa, molecular sieves 4 Angstroem / 24 h / Ambient temperature 2: 100 percent / aq. HCl / tetrahydrofuran; H2O / 3 h / 60 °C View Scheme |

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 93 percent / i-PrNEt / CH2Cl2 / 3 h / Ambient temperature 2: 92 percent / aq. K2CO3 / methanol / 2 h / Ambient temperature 3: 84 percent / pyridinium chlorochromate, AcONa, molecular sieves 4 Angstroem / 24 h / Ambient temperature 4: 100 percent / aq. HCl / tetrahydrofuran; H2O / 3 h / 60 °C View Scheme |

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 92 percent / aq. K2CO3 / methanol / 2 h / Ambient temperature 2: 84 percent / pyridinium chlorochromate, AcONa, molecular sieves 4 Angstroem / 24 h / Ambient temperature 3: 100 percent / aq. HCl / tetrahydrofuran; H2O / 3 h / 60 °C View Scheme |

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: pyridine / 3 h / Ambient temperature 2: 93 percent / i-PrNEt / CH2Cl2 / 3 h / Ambient temperature 3: 92 percent / aq. K2CO3 / methanol / 2 h / Ambient temperature 4: 84 percent / pyridinium chlorochromate, AcONa, molecular sieves 4 Angstroem / 24 h / Ambient temperature 5: 100 percent / aq. HCl / tetrahydrofuran; H2O / 3 h / 60 °C View Scheme | |
| Multi-step reaction with 2 steps 1: I2, CaCO3 / methanol; H2O / 24 h / Ambient temperature 2: 100 percent / aq. HCl / tetrahydrofuran; H2O / 3 h / 60 °C View Scheme |
-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

-
-
124623-17-2
2-amino-5-methylthiopropiophenone

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 24h; Heating; | 99% |
-
-
1609324-12-0
1-(2-amino-phenyl)-3-trimethylsilanyl-propan-1-one

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid; acetic acid In toluene at 100℃; for 20h; | 98% |
-
-
1609257-42-2
2-(3'-trimethylsilylpropionyl)-N-Boc-aniline

-
-
110351-94-5
(4S)-4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid; acetic acid In toluene at 100℃; for 24h; | 96% |
Related products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View