Dayang Chem (Hangzhou) Co.,Ltd.
As a leading manufacturer and supplier of chemicals in China, DayangChem not only supply popular chemicals, but also DayangChem’s R&D center offer custom synthesis according to the contract research and development services for the fine chemicals, ph
Qingdao Beluga Import and Export Co., LTD
Monatin CAS:146142-94-1 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organic intermediates, s
Cas:146142-94-1
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Henan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Zibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Henan Tianfu Chemical Co., Ltd.
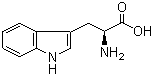
Monatin Basic information Product Name: Monatin Synonyms: Monatin;(2S,4S)-2-Amino-4-carboxy-4-hydroxy-5-(3-indolyl)pentanoic acid CAS: 146142-94-1 MF: C14H16N2O5 MW: 292.29 EINECS: -0 Product Categories: Mol File: Mol File Appearance:Sol
Cas:146142-94-1
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquirySuzhou Health Chemicals Co., Ltd.
High quality,stable supply chain.Appearance:white/off-white or light yellow Storage:Store in cool and dry place, keep away from strong light and heat. Package:aluminum bottle,glass bottle,PTFE bottle,cardboard drum Application:This product can be use
Xi`an Eastling Biotech Co., Ltd.
high purity lowest priceAppearance:solid or liquid Storage:in sealed air resistant place Package:Foil bag; Drum; Plastic bottle Application:Pharma;Industry;Agricultural Transportation:by sea or air Port:Beijing or Guangzhou
Xian Changyue Biological Technology Co., Ltd.
best seller Application:API
Antimex Chemical Limied
Ansciep Chemical is a professional enterprise manufacturing and distributing fine chemicals and speciality chemicals. We have been dedicated to heterocycle compounds and phenyl rings for tens of years. This is our mature product for export. Our quali
Wuhan Circle Star Chem-medical Technology co.,Ltd.
good quality, competitive price, thoughtful after sale serviceAppearance:White to Off-White Solid Storage:Keep it in dry,shady and cool place Package:as your requirement Application:Pharma;Industry;Agricultural;chemical reaserch Transportation:by Sea
DB BIOTECH CO., LTD
best seller Application:API
Henan Kanbei Chemical Co.,LTD
factory?direct?saleAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:healing drugs Transportation:By sea Port:Shanghai/tianjin
Shanghai Chinqesen Biotechnology Co., Ltd.
Good Quality Package:1kg/bag Application:Medical or chemical Transportation:Air/Train/Sea Port:Shenzhen
Hebei Muhuang Technology Co., Ltd
best seller Application:API
ZHEJIANG JIUZHOU CHEM CO.,LTD
factory?direct?saleAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:healing drugs Transportation:By sea Port:Shanghai/tianjin
Chungking Joyinchem Co., Ltd.
Joyinchem have been committed to chemical supply for several years and have built good cooperation records with multinational chemical corporations and importers from all over the world. Our services include:-Spot goods-Contract manufacturing-Custom
Nanjing Raymon Biotech Co., Ltd.
(2s,4s)-4-amino-2-hydroxy-2-(1h-indol-3-ylmethyl)pentanedioic Acid Storage:keep in dry and cool condition Package:25kg or according to cutomer's demand Application:Chemical research/pharma intermediate Transportation:By Sea,by Air,By courier like DHL
Cas:146142-94-1
Min.Order:0
Negotiable
Type:Trading Company
inquiryLupin Pharmaceuticals ,Inc.
146142-94-1
Synthetic route

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol | 92% |
| With sodium hydroxide In methanol; water at 20℃; for 6h; | 54 mg |
| Stage #1: (2S,4S)-4-Amino-2-(1H-indol-3-ylmethyl)-5-oxo-tetrahydro-furan-2-carboxylic acid With water; potassium hydroxide In methanol at 25℃; for 1h; Inert atmosphere; Large scale reaction; Stage #2: With acetic acid In methanol pH=6.5; Large scale reaction; Stage #3: With acetic acid In methanol pH=6.5; Large scale reaction; optical yield given as %ee; | 193 g |
-
-
855433-72-6
4-amino-2-hydroxy-2-(1H-indol-3-ylmethyl)-pentanedioic acid

-
A
-
400769-77-9
(2S,4R) 4-hydroxy-4-(3-indolylmethyl)-2-aminoglutaric acid

-
B
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| RP18-HPLC resolution; |
-
-
551958-84-0
4-hydroxy-4-(3-indolylmethyl)-2-ketoglutaric acid

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| With pyridoxal 5'-phosphate; tris hydrochloride; monosodium glutamate; Aeromonas hydrophila IFO3820 In water; toluene at 30℃; for 16h; pH=7.6; Product distribution / selectivity; Enzymatic reaction; Culture medium; | |
| With pyridoxal 5'-phosphate; tris hydrochloride; monosodium glutamate; Agrobacterium tumefaciens IFO3058 In water; toluene at 30℃; for 16h; pH=7.6; Product distribution / selectivity; Enzymatic reaction; Culture medium; | |
| With pyridoxal 5'-phosphate; tris hydrochloride; monosodium glutamate; Alcaligenes faecalis ATCC8750 In water; toluene at 30℃; for 16h; pH=7.6; Product distribution / selectivity; Enzymatic reaction; Culture medium; |
-
-
73-22-3
L-Tryptophan

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| With potassium phosphate; pyridoxal 5'-phosphate; magnesium chloride; Comamonas testosteroni proA Aldolase; HIS6-HEXaspC aminotransferase In water for 27 - 29h; pH=7.8; Product distribution / selectivity; Enzymatic reaction; | |
| With potassium phosphate; pyridoxal 5'-phosphate; magnesium chloride; Comamonas testosteroni proA Aldolase; HIS6-HEXaspC aminotransferase In water for 27 - 29h; pH=7.8; Product distribution / selectivity; Enzymatic reaction; |
-
-
73-22-3
L-Tryptophan

-
A
-
914081-89-3
(S)-2-hydroxy-2-(1H-indol-3-ylmethyl)-4-oxo-pentanedioic acid

-
B
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| With potassium phosphate; pyridoxal 5'-phosphate; magnesium chloride; Comamonas testosteroni proA Aldolase; HIS6-HEXaspC aminotransferase In water at 30℃; for 3 - 5h; pH=7.5 - 7.8; Product distribution / selectivity; Enzymatic reaction; |
-
-
56-41-7
L-alanin

-
A
-
914081-89-3
(S)-2-hydroxy-2-(1H-indol-3-ylmethyl)-4-oxo-pentanedioic acid

-
B
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| Stage #1: L-Tryptophan With potassium phosphate; pyridoxal 5'-phosphate; magnesium chloride; Comamonas testosteroni proA Aldolase; HIS6-HEXaspC aminotransferase In water at 30℃; for 3 - 5h; pH=7.5 - 7.8; Enzymatic reaction; Stage #2: L-alanin; HIS6-HEXaspC aminotransferase In water at 30℃; pH=7.5 - 7.8; Product distribution / selectivity; Enzymatic reaction; |
-
-
113-24-6
sodium pyruvate

-
-
73-22-3
L-Tryptophan

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| With potassium phosphate; pyridoxal 5'-phosphate; magnesium chloride; Comamonas testosteroni proA Aldolase; HIS6-HEXaspC aminotransferase In water at 30℃; for 21 - 29h; pH=7.8; Product distribution / selectivity; Enzymatic reaction; |

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 15.6 mg / NaHCO3 / acetonitrile; H2O / 24 h / 20 °C 2: 69 percent / PDC / dimethylformamide / 24 h / 20 °C 3: HCO2H; HCl / 4 h / 20 °C 4: 54 mg / NaOH / methanol; H2O / 6 h / 20 °C View Scheme |
-
-
645396-51-6
2-[(indol-3-yl)methyl]prop-2-en-1-ol

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: CH2Cl2 / 360 h / 20 °C 2: 97 percent / imidaziole / dimethylformamide / 3 h / 20 °C 3: 97 percent / DMAP / acetonitrile / 1 h / 20 °C 4: 100 percent / pyridine*HF / tetrahydrofuran / 1 h / 0 °C 5: H2 / Pd(OH)2/C / methanol / 5 h / 20 °C / atmospheric pressure 6: 15.6 mg / NaHCO3 / acetonitrile; H2O / 24 h / 20 °C 7: 69 percent / PDC / dimethylformamide / 24 h / 20 °C 8: HCO2H; HCl / 4 h / 20 °C 9: 54 mg / NaOH / methanol; H2O / 6 h / 20 °C View Scheme | |
| Multi-step reaction with 9 steps 1: 98 percent / MgBr2*OEt2 / CH2Cl2 / 84 h / 20 °C 2: 97 percent / imidaziole / dimethylformamide / 3 h / 20 °C 3: 97 percent / DMAP / acetonitrile / 1 h / 20 °C 4: 100 percent / pyridine*HF / tetrahydrofuran / 1 h / 0 °C 5: H2 / Pd(OH)2/C / methanol / 5 h / 20 °C / atmospheric pressure 6: 15.6 mg / NaHCO3 / acetonitrile; H2O / 24 h / 20 °C 7: 69 percent / PDC / dimethylformamide / 24 h / 20 °C 8: HCO2H; HCl / 4 h / 20 °C 9: 54 mg / NaOH / methanol; H2O / 6 h / 20 °C View Scheme | |
| Multi-step reaction with 9 steps 1: CH2Cl2 / 360 h / 20 °C 2: 97 percent / imidazole / dimethylformamide 3: 97 percent / DMAP / acetonitrile 4: 100 percent / HF*pyridine / tetrahydrofuran 5: H2 / Pd(OH)2/C / methanol 6: acetonitrile 7: 69 percent / PDC / dimethylformamide 8: HCl; HCOOH 9: 92 percent / NaOH / methanol View Scheme | |
| Multi-step reaction with 9 steps 1: 98 percent / MgBr2*OEt2 / CH2Cl2 / 72 h / 20 °C 2: 97 percent / imidazole / dimethylformamide 3: 97 percent / DMAP / acetonitrile 4: 100 percent / HF*pyridine / tetrahydrofuran 5: H2 / Pd(OH)2/C / methanol 6: acetonitrile 7: 69 percent / PDC / dimethylformamide 8: HCl; HCOOH 9: 92 percent / NaOH / methanol View Scheme |
-
-
87438-91-3
ethyl 2-((1H-indol-3-yl)methyl)acrylate

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 85 percent / DIBAL-H / CH2Cl2; hexane / -78 - 20 °C 2: CH2Cl2 / 360 h / 20 °C 3: 97 percent / imidaziole / dimethylformamide / 3 h / 20 °C 4: 97 percent / DMAP / acetonitrile / 1 h / 20 °C 5: 100 percent / pyridine*HF / tetrahydrofuran / 1 h / 0 °C 6: H2 / Pd(OH)2/C / methanol / 5 h / 20 °C / atmospheric pressure 7: 15.6 mg / NaHCO3 / acetonitrile; H2O / 24 h / 20 °C 8: 69 percent / PDC / dimethylformamide / 24 h / 20 °C 9: HCO2H; HCl / 4 h / 20 °C 10: 54 mg / NaOH / methanol; H2O / 6 h / 20 °C View Scheme | |
| Multi-step reaction with 10 steps 1: 85 percent / DIBAL-H / CH2Cl2; hexane / -78 - 20 °C 2: 98 percent / MgBr2*OEt2 / CH2Cl2 / 84 h / 20 °C 3: 97 percent / imidaziole / dimethylformamide / 3 h / 20 °C 4: 97 percent / DMAP / acetonitrile / 1 h / 20 °C 5: 100 percent / pyridine*HF / tetrahydrofuran / 1 h / 0 °C 6: H2 / Pd(OH)2/C / methanol / 5 h / 20 °C / atmospheric pressure 7: 15.6 mg / NaHCO3 / acetonitrile; H2O / 24 h / 20 °C 8: 69 percent / PDC / dimethylformamide / 24 h / 20 °C 9: HCO2H; HCl / 4 h / 20 °C 10: 54 mg / NaOH / methanol; H2O / 6 h / 20 °C View Scheme | |
| Multi-step reaction with 11 steps 1: 97 percent / Et3N; 4-DMAP / CH2Cl2 / 20 °C 2: 72 percent / DIBAL-H / CH2Cl2 / -78 °C 3: MgBr2*OEt2 / CH2Cl2 / 96 h / 20 °C 4: 97 percent / imidazole / dimethylformamide 5: 97 percent / DMAP / acetonitrile 6: 100 percent / HF*pyridine / tetrahydrofuran 7: H2 / Pd(OH)2/C / methanol 8: acetonitrile 9: 69 percent / PDC / dimethylformamide 10: HCl; HCOOH 11: 92 percent / NaOH / methanol View Scheme |
-
-
645396-53-8
(2S,5S,8aS)-2-(hydroxymethyl)-2-[(indol-3-yl)methyl]-5-phenyl-1,5,6,8a-tetrahydro-3,7-dioxaindolizin-8(2H)-one

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 97 percent / imidaziole / dimethylformamide / 3 h / 20 °C 2: 97 percent / DMAP / acetonitrile / 1 h / 20 °C 3: 100 percent / pyridine*HF / tetrahydrofuran / 1 h / 0 °C 4: H2 / Pd(OH)2/C / methanol / 5 h / 20 °C / atmospheric pressure 5: 15.6 mg / NaHCO3 / acetonitrile; H2O / 24 h / 20 °C 6: 69 percent / PDC / dimethylformamide / 24 h / 20 °C 7: HCO2H; HCl / 4 h / 20 °C 8: 54 mg / NaOH / methanol; H2O / 6 h / 20 °C View Scheme | |
| Multi-step reaction with 8 steps 1: 97 percent / imidazole / dimethylformamide 2: 97 percent / DMAP / acetonitrile 3: 100 percent / HF*pyridine / tetrahydrofuran 4: H2 / Pd(OH)2/C / methanol 5: acetonitrile 6: 69 percent / PDC / dimethylformamide 7: HCl; HCOOH 8: 92 percent / NaOH / methanol View Scheme |
-
-
645396-57-2
(2S,4S)-4-(tert-butoxycarbonyl)amino-2-[(1-(tert-butoxycarbonyl)indol-3-yl)methyl]-2-(hydroxymethyl)oxolan-5-one

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 69 percent / PDC / dimethylformamide / 24 h / 20 °C 2: HCO2H; HCl / 4 h / 20 °C 3: 54 mg / NaOH / methanol; H2O / 6 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 69 percent / PDC / dimethylformamide 2: HCl; HCOOH 3: 92 percent / NaOH / methanol View Scheme |
-
-
645396-58-3
(2S,4S)-4-(tert-butyloxycarbonyl)amino-2-[(1-(tert-butyloxycarbonyl)indol-3-yl)methyl]-5-oxooxolane-2-carboxylic acid

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: HCO2H; HCl / 4 h / 20 °C 2: 54 mg / NaOH / methanol; H2O / 6 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: HCl; HCOOH 2: 92 percent / NaOH / methanol View Scheme |
-
-
645396-59-4
(2S,5S,8aS)-2-[(tert-butyl(dimethyl)silyloxy)methyl]-2-[(indol-3-yl)methyl]-2-(hydroxymethyl)-5-phenyl-1,5,6,8a-tetrahydro-3,7-dioxaindolizin-8(2H)-one

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 97 percent / DMAP / acetonitrile / 1 h / 20 °C 2: 100 percent / pyridine*HF / tetrahydrofuran / 1 h / 0 °C 3: H2 / Pd(OH)2/C / methanol / 5 h / 20 °C / atmospheric pressure 4: 15.6 mg / NaHCO3 / acetonitrile; H2O / 24 h / 20 °C 5: 69 percent / PDC / dimethylformamide / 24 h / 20 °C 6: HCO2H; HCl / 4 h / 20 °C 7: 54 mg / NaOH / methanol; H2O / 6 h / 20 °C View Scheme | |
| Multi-step reaction with 7 steps 1: 97 percent / DMAP / acetonitrile 2: 100 percent / HF*pyridine / tetrahydrofuran 3: H2 / Pd(OH)2/C / methanol 4: acetonitrile 5: 69 percent / PDC / dimethylformamide 6: HCl; HCOOH 7: 92 percent / NaOH / methanol View Scheme |
-
-
645396-54-9
(2S,5S,8aS)-2-[(1-(tertbutoxycarbonyl)indol-3-yl)methyl]-2-(hydroxymethyl)-5-phenyl-1,5,6,8a-tetrahydro-3,7-dioxaindolizin-8(2H)-one

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: H2 / Pd(OH)2/C / methanol / 5 h / 20 °C / atmospheric pressure 2: 15.6 mg / NaHCO3 / acetonitrile; H2O / 24 h / 20 °C 3: 69 percent / PDC / dimethylformamide / 24 h / 20 °C 4: HCO2H; HCl / 4 h / 20 °C 5: 54 mg / NaOH / methanol; H2O / 6 h / 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1: H2 / Pd(OH)2/C / methanol 2: acetonitrile 3: 69 percent / PDC / dimethylformamide 4: HCl; HCOOH 5: 92 percent / NaOH / methanol View Scheme |
-
-
645396-60-7
(2S,5S,8aS)-2-[(tert-butyl(dimethyl)silyloxy)methyl]-2-[(1-(tert-butoxycarbonyl)indol-3-yl)methyl]-2-(hydroxymethyl)-5-phenyl-1,5,6,8a-tetrahydro-3,7-dioxaindolizin-8(2H)-one

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 100 percent / pyridine*HF / tetrahydrofuran / 1 h / 0 °C 2: H2 / Pd(OH)2/C / methanol / 5 h / 20 °C / atmospheric pressure 3: 15.6 mg / NaHCO3 / acetonitrile; H2O / 24 h / 20 °C 4: 69 percent / PDC / dimethylformamide / 24 h / 20 °C 5: HCO2H; HCl / 4 h / 20 °C 6: 54 mg / NaOH / methanol; H2O / 6 h / 20 °C View Scheme | |
| Multi-step reaction with 6 steps 1: 100 percent / HF*pyridine / tetrahydrofuran 2: H2 / Pd(OH)2/C / methanol 3: acetonitrile 4: 69 percent / PDC / dimethylformamide 5: HCl; HCOOH 6: 92 percent / NaOH / methanol View Scheme |
-
-
855436-27-0
(3S)-5-(3-indolylmethyl)-4,5-dihydroisoxazole-3,5-dicarboxylic acid

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / Na/Hg / ethanol 2: RP18-HPLC resolution View Scheme |
-
-
855433-63-5
ethyl (3S)-5-(3-indolylmethyl)-5-carboxy-4,5-dihydroisoxazole-3-carboxylate

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: KOH / ethanol; H2O / 2.5 h / 20 °C 2: 60 percent / Na/Hg / ethanol 3: RP18-HPLC resolution View Scheme |

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: Aspergillus oryzae protease; aq. NaOH / aq. phosphate buffer; toluene / pH 7.0 2: KOH / ethanol; H2O / 2.5 h / 20 °C 3: 60 percent / Na/Hg / ethanol 4: RP18-HPLC resolution View Scheme | |
| Multi-step reaction with 5 steps 1.1: hydrogen; potassium hydroxide / ethanol / 1.5 h / 21 °C / 1500.15 - 3750.38 Torr / Sealed reactor; Large scale reaction 2.1: hydrogenchloride / methanol / 20 - 45 °C / Large scale reaction 2.2: 1.5 h / 5 - 25 °C / Large scale reaction 2.3: 15 h / 25 °C / Large scale reaction 3.1: protease from Bacillus lichenformis ChiroCLEC-BL / acetonitrile / 19 h / 22 °C / pH 7.5 / aq. phosphate buffer; Large scale reaction; Enzymatic reaction 4.1: palladium 10% on activated carbon; hydrogen; triethylamine / methanol / 1 h / 40 °C / 2250.23 Torr / Sealed vessel; Large scale reaction 5.1: water; potassium hydroxide / methanol / 1 h / 25 °C / Inert atmosphere; Large scale reaction 5.2: pH 6.5 / Large scale reaction 5.3: pH 6.5 / Large scale reaction View Scheme |
-
-
645396-52-7
3-(2-hydroxymethyl-allyl)-indole-1-carboxylic acid tert-butyl ester

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: MgBr2*OEt2 / CH2Cl2 / 96 h / 20 °C 2: 97 percent / imidazole / dimethylformamide 3: 97 percent / DMAP / acetonitrile 4: 100 percent / HF*pyridine / tetrahydrofuran 5: H2 / Pd(OH)2/C / methanol 6: acetonitrile 7: 69 percent / PDC / dimethylformamide 8: HCl; HCOOH 9: 92 percent / NaOH / methanol View Scheme | |
| Multi-step reaction with 6 steps 1: MgBr2*OEt2 / CH2Cl2 / 96 h / 20 °C 2: H2 / Pd(OH)2/C / methanol 3: acetonitrile 4: 69 percent / PDC / dimethylformamide 5: HCl; HCOOH 6: 92 percent / NaOH / methanol View Scheme |
-
-
645396-50-5
3-(2-ethoxycarbonyl-allyl)-indole-1-carboxylic acid tert-butyl ester

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 72 percent / DIBAL-H / CH2Cl2 / -78 °C 2: MgBr2*OEt2 / CH2Cl2 / 96 h / 20 °C 3: 97 percent / imidazole / dimethylformamide 4: 97 percent / DMAP / acetonitrile 5: 100 percent / HF*pyridine / tetrahydrofuran 6: H2 / Pd(OH)2/C / methanol 7: acetonitrile 8: 69 percent / PDC / dimethylformamide 9: HCl; HCOOH 10: 92 percent / NaOH / methanol View Scheme | |
| Multi-step reaction with 7 steps 1: 72 percent / DIBAL-H / CH2Cl2 / -78 °C 2: MgBr2*OEt2 / CH2Cl2 / 96 h / 20 °C 3: H2 / Pd(OH)2/C / methanol 4: acetonitrile 5: 69 percent / PDC / dimethylformamide 6: HCl; HCOOH 7: 92 percent / NaOH / methanol View Scheme |

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: acetonitrile 2: 69 percent / PDC / dimethylformamide 3: HCl; HCOOH 4: 92 percent / NaOH / methanol View Scheme |
-
-
400769-77-9
(2S,4R) 4-hydroxy-4-(3-indolylmethyl)-2-aminoglutaric acid

-
-
400769-79-1
(2S,4R)-monatin

-
A
-
400769-81-5
(2R,4R)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

-
B
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| With salicylaldehyde; acetic acid In methanol at 65℃; for 143h; pH=5; Product distribution / selectivity; |


-
A
-
400769-81-5
(2R,4R)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

-
B
-
400769-77-9
(2S,4R) 4-hydroxy-4-(3-indolylmethyl)-2-aminoglutaric acid

-
C
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

-
D
-
400769-79-1
(2S,4R)-monatin

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen; 5% rhodium-on-charcoal In water at 25℃; under 7500.75 Torr; for 24h; Product distribution / selectivity; |


-
A
-
400769-81-5
(2R,4R)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

-
B
-
400769-77-9
(2S,4R) 4-hydroxy-4-(3-indolylmethyl)-2-aminoglutaric acid

-
C
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| With methanol; salicylaldehyde; acetic acid at 80℃; for 16h; Product distribution / selectivity; |


-
A
-
400769-81-5
(2R,4R)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

-
B
-
400769-77-9
(2S,4R) 4-hydroxy-4-(3-indolylmethyl)-2-aminoglutaric acid

-
C
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

-
D
-
400769-79-1
(2S,4R)-monatin

| Conditions | Yield |
|---|---|
| Stage #1: isoxazoline diethyl ester With potassium hydroxide; water; sponge nickel catalyst A-7063 In ethanol for 0.0833333h; Sealed reactor; Stage #2: With hydrogen In ethanol; water at 20℃; under 3750.38 Torr; for 1.5h; Product distribution / selectivity; Sealed reactor; | |
| Stage #1: isoxazoline diethyl ester With potassium hydroxide; water In ethanol at 20℃; Stage #2: With hydrogen; sponge nickel catalyst A5200 In ethanol; water at 20℃; under 3750.38 Torr; for 2h; Product distribution / selectivity; | |
| Stage #1: isoxazoline diethyl ester With potassium hydroxide; water In ethanol at 20℃; Stage #2: With hydrogen; 5%-palladium/activated carbon In ethanol; water at 20℃; under 3750.38 Torr; for 2h; Product distribution / selectivity; |


-
A
-
400769-81-5
(2R,4R)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

-
B
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| Stage #1: isoxazoline diethyl ester With potassium hydroxide; water; sponge nickel catalyst A-7063 In ethanol at 21℃; for 0.0833333h; Sealed reactor; Stage #2: With hydrogen In ethanol under 1500.15 - 3750.38 Torr; for 1.41667h; Sealed reactor; Stage #3: With acetic acid In ethanol at 20℃; for 15h; pH=4.5; Product distribution / selectivity; |

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| With hydrogen; palladium 10% on activated carbon In methanol for 3h; Product distribution / selectivity; |
-
-
57-60-3
piruvate

-
-
73-22-3
L-Tryptophan

-
A
-
551958-84-0
4-hydroxy-4-(3-indolylmethyl)-2-ketoglutaric acid

-
B
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| Stage #1: piruvate; L-Tryptophan With magnesium chloride; pyridoxal 5'-phosphate at 30℃; pH=7.5 - 7.8; Inert atmosphere; phosphate buffer; Enzymatic reaction; Stage #2: With L-alanin at 30℃; pH=7.5 - 7.8; Product distribution / selectivity; Inert atmosphere; Enzymatic reaction; |

-
-
535967-29-4
methyl 2-((1H-indol-3-yl)methyl)-4-(benzyloxycarbonylamino)-5-oxotetrahydrofuran-2-carboxylate

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: palladium 10% on activated carbon; hydrogen; triethylamine / methanol / 1 h / 40 °C / 2250.23 Torr / Sealed vessel; Large scale reaction 2.1: water; potassium hydroxide / methanol / 1 h / 25 °C / Inert atmosphere; Large scale reaction 2.2: pH 6.5 / Large scale reaction 2.3: pH 6.5 / Large scale reaction View Scheme |
-
-
70-34-8
2,4-Dinitrofluorobenzene

-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In ethanol; water Ambient temperature; |
-
-
146142-94-1
(2S,4S)-2-((1H-indol-3-yl)methyl)-4-amino-2-hydroxypentane-1,5-dioic acid

-
-
146029-61-0
Methyl (2S,4S)-2-(indol-3-ylmethyl)-4-(2,4-dinitroanilino)-5-oxo-2,3,4,5-tetrahydrofuran-2-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydrogen carbonate / ethanol; H2O / Ambient temperature 2: diethyl ether / 0.5 h View Scheme |
Related products
Raw Materials
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View