Shandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:146436-22-8
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHebei Nengqian Chemical Import and Export Co., LTD
With our good experience, we offer detailed technical support and advice to assist customers. We communicate closely with customers to establish their quality requirements. Consistent Quality Our plant has strict quality control in each manufacturin
Cas:146436-22-8
Min.Order:1 Kilogram
FOB Price: $1.0 / 10.0
Type:Trading Company
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:146436-22-8
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryXiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
KAISA GROUP INC
1.Applied in food field.it can improve the immune system and prolong life. 2.Appliedin cosmetic field.it can improve the skin care. 3.Applied in pharmaceutical field.it can treat various dieases. 4.Our product quality assurance will make our customer
Cas:146436-22-8
Min.Order:1 Metric Ton
FOB Price: $7.0 / 8.0
Type:Trading Company
inquiryXi`an Eastling Biotech Co., Ltd.
high purity lowest priceAppearance:solid or liquid Storage:in sealed air resistant place Package:Foil bag; Drum; Plastic bottle Application:Pharma;Industry;Agricultural Transportation:by sea or air Port:Beijing or Guangzhou
Henan Tianfu Chemical Co., Ltd.
1.Our services:A.Supply sampleB.The packing also can be according the customers` requirmentC.Any inquiries will be replied within 24 hoursD.we provide Commerical Invoice, Packing List, Bill of loading, COA , Health certificate and Origin certificate.
Cas:146436-22-8
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryAntimex Chemical Limied
Ansciep Chemical is a professional enterprise manufacturing and distributing fine chemicals and speciality chemicals. We have been dedicated to heterocycle compounds and phenyl rings for tens of years. This is our mature product for export. Our quali
Cas:146436-22-8
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHANGZHOU TIANYE CHEMICALS CO., LTD.
We product this chemical more than 10 years . We are very experience to export it to many countries, Our superior & stable quality , competitive price gain warm reception from our customers. Application:Chemical intermediate
Cas:146436-22-8
Min.Order:0
Negotiable
Type:Trading Company
inquiryGuangdong Juda Chemical Industrial Co.,Limited
Factory supply high purity low priceAppearance:solid or liquid Storage:sealed in cool and dry place Package:As customer's requested Application:Pharma Intermediate Transportation:by courier/air/sea Port:Any port in China
Changchun Artel lmport and Export trade company
Supply top quality products with a reasonable price Application:api
Henan Kanbei Chemical Co.,LTD
factory?direct?saleAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:healing drugs Transportation:By sea Port:Shanghai/tianjin
Shanghai Chinqesen Biotechnology Co., Ltd.
Good Quality Package:1kg/bag Application:Medical or chemical Transportation:Air/Train/Sea Port:Shenzhen
ZHEJIANG JIUZHOU CHEM CO.,LTD
factory?direct?saleAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:healing drugs Transportation:By sea Port:Shanghai/tianjin
Jilin haofei import and export trade Co.,Ltd
Price, service, company and transport advantage: 1. Best service, place of origin China, high quality, and reasonable price. 2. It's customers' right to choose the package (EMS, DHL, FEDEX, UPS). 3. It's customers' right
Shanghai AngewChem Co., Ltd.
99%, in stock Shanghai AngewChemCo., Ltd. is an innovative enterprise on fine chemicals and pharmaceuticals. Based on Shanghai R&D center and Hunan chemical manufacturing plant, we offer chemical research, process development, and large-scale
Cas:146436-22-8
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShanghai Forever Synthesis Co.,Ltd.
As a professional chemical R&D and synthesis supplier, Shanghai Forever Synthesis Co.,Ltd. is specialized R&D and synthesizing APIs and Impurities. Besides, we value more special service, that is, to customize chemical synthesis according to client’s
Chengdu Push Bio-technology Co., Ltd
Chengdu Push Bio-technology Co., Ltd. provides more than 4,000 natural monomeric substances, including approximately 6,000 products at different grades, specifications and purities. We have specialized in the R&D of medicinally active ingredients sta
Henan Wising Chem Co., Ltd
Henan Wising Chem specialize in sourcing the chemicals in China. We are associated with many trusted manufacturers each having different area of expertise required for meeting the needs of our local as well as overseas buyers . We have access to cu
Cas:146436-22-8
Min.Order:10 Milligram
Negotiable
Type:Lab/Research institutions
inquiryWuhan MoonZY Biological Technology Co.,Ltd
instock with good quality and wholesale price Storage:Keep in a cool & dry place Package:Packing material and QTY as your request Application:Pharma;Industry;other application Transportation:Express or as your request Port:Any port of China
Cas:146436-22-8
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryNanjing Raymon Biotech Co., Ltd.
terpestacin Storage:Store in dry and cool condition Package:25kg or according to cutomer's demand Application:Chemical research/Pharmaceutical intermediates Transportation:By Sea,by Air,By courier like DHL or Fedx. Port:Shanghai/Shenzhen
Finetech Industry Limited
High Quality Best Price Storage:Store in dry, dark and ventilated place Application:Chemical Synthesis Intermediate
Synthetic route
-
-
429621-48-7
(5E,10E,14E)-(3aR,7S,16aS)-7-(tert-Butyl-dimethyl-silanyloxy)-2-hydroxy-3-((S)-2-hydroxy-1-methyl-ethyl)-6,10,14,16a-tetramethyl-4,7,8,9,12,13,16,16a-octahydro-3aH-cyclopentacyclopentadecen-1-one

-
-
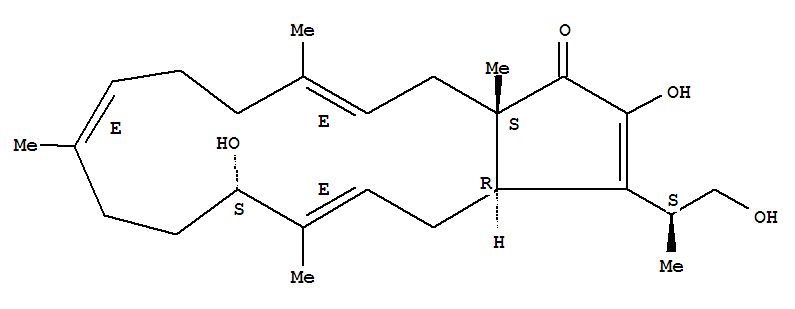
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran | 94% |
-
-
1385030-65-8
C37H66O4Si2

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| With hydrogen fluoride In tetrahydrofuran; water at 20℃; for 3h; | 90% |
-
-
935263-74-4
7-hydroxy-3-(2-hydroxy-1-methyl-ethyl)-2-(4-methoxy-benzyloxy)-6,10,14,16a-tetramethyl-4,7,8,9,12,13,16,16a-octahydro-3aH-cyclopentacyclopentadecen-1-one

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| With dimethylsulfide; magnesium bromide In dichloromethane at 20℃; for 0.666667h; | 74% |
| With dimethylsulfide; magnesium bromide ethyl etherate In dichloromethane at -78 - 20℃; Inert atmosphere; | 74% |

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; water at 50℃; for 1h; Hydrolysis; deprotection; | 70% |

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol at 20℃; for 2h; | 2.5 mg |
-
-
935263-58-4
8-benzenesulfonyl-2,6-dimethyl-octa-1,6-dien-3-ol

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1: 85 percent / LiHMDS; HMPA / tetrahydrofuran / 0.08 h / -40 °C 2: 77 percent / NaBH4; DPPP / Pd(OAc)2 / dimethylsulfoxide 3: 44 percent / Grubbs second generation catalyst / benzene / 16 h / 20 °C 4: 93 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C 5: (S,S)-N-[2-(2-PPh2-benzoylamino)cyclohexyl]-2-PPh2-benzamide / Pd2dba3*CHCl3 / CH2Cl2 / 20 °C 6: 1,2-dimethoxy-ethane / 1 h / 150 °C / microwave irradiation 7: Cs2CO3; Bu4NI / dimethylformamide / 2 h 8: 8.7 mg / pyridine / 3 h / 20 °C 9: 65 percent / AD-α-mix; CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 48 h / 4 °C 10: NaIO4 / tetrahydrofuran; H2O / 1 h / 20 °C 11: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 12: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 13: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |
-
-
935263-72-2
[3-(5-benzenesulfonyl-3-methyl-pent-3-enyl)-2-methyl-oxiranyl]-methanol

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1.1: pyridine; iodine; PPh3 / acetonitrile; diethyl ether / 2 h / 0 °C 1.2: 74 percent / water / acetonitrile; diethyl ether / 8 h / 38 °C 2.1: 85 percent / LiHMDS; HMPA / tetrahydrofuran / 0.08 h / -40 °C 3.1: 77 percent / NaBH4; DPPP / Pd(OAc)2 / dimethylsulfoxide 4.1: 44 percent / Grubbs second generation catalyst / benzene / 16 h / 20 °C 5.1: 93 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C 6.1: (S,S)-N-[2-(2-PPh2-benzoylamino)cyclohexyl]-2-PPh2-benzamide / Pd2dba3*CHCl3 / CH2Cl2 / 20 °C 7.1: 1,2-dimethoxy-ethane / 1 h / 150 °C / microwave irradiation 8.1: Cs2CO3; Bu4NI / dimethylformamide / 2 h 9.1: 8.7 mg / pyridine / 3 h / 20 °C 10.1: 65 percent / AD-α-mix; CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 48 h / 4 °C 11.1: NaIO4 / tetrahydrofuran; H2O / 1 h / 20 °C 12.1: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 13.1: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 14.1: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |
-
-
935263-61-9
5-methyl-5-(3-methyl-4-triisopropylsilanyloxy-but-2-enyl)-cyclopent-3-ene-1,2-dione

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 17 steps 1: MgBr2*Et2O / CH2Cl2 / 1 h / 20 °C 2: 79 percent / Cs2CO3; Bu4NI / dimethylformamide / 2.5 h / 20 °C 3: 84 percent / TBAF*3H2O / tetrahydrofuran / 2 h / 0 °C 4: 88 percent / PPh3; CBr4 / acetonitrile / 20 °C 5: 85 percent / LiHMDS; HMPA / tetrahydrofuran / 0.08 h / -40 °C 6: 77 percent / NaBH4; DPPP / Pd(OAc)2 / dimethylsulfoxide 7: 44 percent / Grubbs second generation catalyst / benzene / 16 h / 20 °C 8: 93 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C 9: (S,S)-N-[2-(2-PPh2-benzoylamino)cyclohexyl]-2-PPh2-benzamide / Pd2dba3*CHCl3 / CH2Cl2 / 20 °C 10: 1,2-dimethoxy-ethane / 1 h / 150 °C / microwave irradiation 11: Cs2CO3; Bu4NI / dimethylformamide / 2 h 12: 8.7 mg / pyridine / 3 h / 20 °C 13: 65 percent / AD-α-mix; CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 48 h / 4 °C 14: NaIO4 / tetrahydrofuran; H2O / 1 h / 20 °C 15: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 16: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 17: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 18 steps 1.1: CHCl3 / 0.5 h / 100 - 120 °C / microwave irradiation 1.2: Cs2CO3 / Pd(OAc)2 / acetonitrile / 0.5 h / 20 °C 2.1: MgBr2*Et2O / CH2Cl2 / 1 h / 20 °C 3.1: 79 percent / Cs2CO3; Bu4NI / dimethylformamide / 2.5 h / 20 °C 4.1: 84 percent / TBAF*3H2O / tetrahydrofuran / 2 h / 0 °C 5.1: 88 percent / PPh3; CBr4 / acetonitrile / 20 °C 6.1: 85 percent / LiHMDS; HMPA / tetrahydrofuran / 0.08 h / -40 °C 7.1: 77 percent / NaBH4; DPPP / Pd(OAc)2 / dimethylsulfoxide 8.1: 44 percent / Grubbs second generation catalyst / benzene / 16 h / 20 °C 9.1: 93 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C 10.1: (S,S)-N-[2-(2-PPh2-benzoylamino)cyclohexyl]-2-PPh2-benzamide / Pd2dba3*CHCl3 / CH2Cl2 / 20 °C 11.1: 1,2-dimethoxy-ethane / 1 h / 150 °C / microwave irradiation 12.1: Cs2CO3; Bu4NI / dimethylformamide / 2 h 13.1: 8.7 mg / pyridine / 3 h / 20 °C 14.1: 65 percent / AD-α-mix; CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 48 h / 4 °C 15.1: NaIO4 / tetrahydrofuran; H2O / 1 h / 20 °C 16.1: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 17.1: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 18.1: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |
-
-
935263-71-1
4-allyl-5-(4-hydroxy-3-methyl-but-2-enyl)-2-(4-methoxy-benzyloxy)-5-methyl-cyclopent-2-enone

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1: 88 percent / PPh3; CBr4 / acetonitrile / 20 °C 2: 85 percent / LiHMDS; HMPA / tetrahydrofuran / 0.08 h / -40 °C 3: 77 percent / NaBH4; DPPP / Pd(OAc)2 / dimethylsulfoxide 4: 44 percent / Grubbs second generation catalyst / benzene / 16 h / 20 °C 5: 93 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C 6: (S,S)-N-[2-(2-PPh2-benzoylamino)cyclohexyl]-2-PPh2-benzamide / Pd2dba3*CHCl3 / CH2Cl2 / 20 °C 7: 1,2-dimethoxy-ethane / 1 h / 150 °C / microwave irradiation 8: Cs2CO3; Bu4NI / dimethylformamide / 2 h 9: 8.7 mg / pyridine / 3 h / 20 °C 10: 65 percent / AD-α-mix; CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 48 h / 4 °C 11: NaIO4 / tetrahydrofuran; H2O / 1 h / 20 °C 12: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 13: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 14: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |
-
-
935263-59-5
4-allyl-5-(4-bromo-3-methyl-but-2-enyl)-2-(4-methoxy-benzyloxy)-5-methyl-cyclopent-2-enone

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1: 85 percent / LiHMDS; HMPA / tetrahydrofuran / 0.08 h / -40 °C 2: 77 percent / NaBH4; DPPP / Pd(OAc)2 / dimethylsulfoxide 3: 44 percent / Grubbs second generation catalyst / benzene / 16 h / 20 °C 4: 93 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C 5: (S,S)-N-[2-(2-PPh2-benzoylamino)cyclohexyl]-2-PPh2-benzamide / Pd2dba3*CHCl3 / CH2Cl2 / 20 °C 6: 1,2-dimethoxy-ethane / 1 h / 150 °C / microwave irradiation 7: Cs2CO3; Bu4NI / dimethylformamide / 2 h 8: 8.7 mg / pyridine / 3 h / 20 °C 9: 65 percent / AD-α-mix; CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 48 h / 4 °C 10: NaIO4 / tetrahydrofuran; H2O / 1 h / 20 °C 11: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 12: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 13: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |
-
-
935263-62-0
4-allyl-2-hydroxy-5-methyl-5-(3-methyl-4-triisopropylsilanyloxy-but-2-enyl)-cyclopent-2-enone

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 16 steps 1: 79 percent / Cs2CO3; Bu4NI / dimethylformamide / 2.5 h / 20 °C 2: 84 percent / TBAF*3H2O / tetrahydrofuran / 2 h / 0 °C 3: 88 percent / PPh3; CBr4 / acetonitrile / 20 °C 4: 85 percent / LiHMDS; HMPA / tetrahydrofuran / 0.08 h / -40 °C 5: 77 percent / NaBH4; DPPP / Pd(OAc)2 / dimethylsulfoxide 6: 44 percent / Grubbs second generation catalyst / benzene / 16 h / 20 °C 7: 93 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C 8: (S,S)-N-[2-(2-PPh2-benzoylamino)cyclohexyl]-2-PPh2-benzamide / Pd2dba3*CHCl3 / CH2Cl2 / 20 °C 9: 1,2-dimethoxy-ethane / 1 h / 150 °C / microwave irradiation 10: Cs2CO3; Bu4NI / dimethylformamide / 2 h 11: 8.7 mg / pyridine / 3 h / 20 °C 12: 65 percent / AD-α-mix; CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 48 h / 4 °C 13: NaIO4 / tetrahydrofuran; H2O / 1 h / 20 °C 14: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 15: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 16: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |
-
-
935263-65-3
2,7-dihydroxy-6,10,14,16a-tetramethyl-4,7,8,9,12,13,16,16a-octahydro-3aH-cyclopentacyclopentadecen-1-one

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: (S,S)-N-[2-(2-PPh2-benzoylamino)cyclohexyl]-2-PPh2-benzamide / Pd2dba3*CHCl3 / CH2Cl2 / 20 °C 2: 1,2-dimethoxy-ethane / 1 h / 150 °C / microwave irradiation 3: Cs2CO3; Bu4NI / dimethylformamide / 2 h 4: 8.7 mg / pyridine / 3 h / 20 °C 5: 65 percent / AD-α-mix; CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 48 h / 4 °C 6: NaIO4 / tetrahydrofuran; H2O / 1 h / 20 °C 7: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 8: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 9: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |
-
-
935263-70-0
4-allyl-2-(4-methoxy-benzyloxy)-5-methyl-5-(3-methyl-4-triisopropylsilanyloxy-but-2-enyl)-cyclopent-2-enone

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 15 steps 1: 84 percent / TBAF*3H2O / tetrahydrofuran / 2 h / 0 °C 2: 88 percent / PPh3; CBr4 / acetonitrile / 20 °C 3: 85 percent / LiHMDS; HMPA / tetrahydrofuran / 0.08 h / -40 °C 4: 77 percent / NaBH4; DPPP / Pd(OAc)2 / dimethylsulfoxide 5: 44 percent / Grubbs second generation catalyst / benzene / 16 h / 20 °C 6: 93 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C 7: (S,S)-N-[2-(2-PPh2-benzoylamino)cyclohexyl]-2-PPh2-benzamide / Pd2dba3*CHCl3 / CH2Cl2 / 20 °C 8: 1,2-dimethoxy-ethane / 1 h / 150 °C / microwave irradiation 9: Cs2CO3; Bu4NI / dimethylformamide / 2 h 10: 8.7 mg / pyridine / 3 h / 20 °C 11: 65 percent / AD-α-mix; CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 48 h / 4 °C 12: NaIO4 / tetrahydrofuran; H2O / 1 h / 20 °C 13: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 14: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 15: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |
-
-
935263-66-4
7-hydroxy-6,10,14,16a-tetramethyl-2-(1-methyl-but-2-enyloxy)-4,7,8,9,12,13,16,16a-octahydro-3aH-cyclopentacyclopentadecen-1-one

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 1,2-dimethoxy-ethane / 1 h / 150 °C / microwave irradiation 2: Cs2CO3; Bu4NI / dimethylformamide / 2 h 3: 8.7 mg / pyridine / 3 h / 20 °C 4: 65 percent / AD-α-mix; CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 48 h / 4 °C 5: NaIO4 / tetrahydrofuran; H2O / 1 h / 20 °C 6: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 7: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 8: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |
-
-
1026157-60-7
2,7-dihydroxy-6,10,14,16a-tetramethyl-3-(1-methyl-but-2-enyl)-4,7,8,9,12,13,16,16a-octahydro-3aH-cyclopentacyclopentadecen-1-one

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: Cs2CO3; Bu4NI / dimethylformamide / 2 h 2: 8.7 mg / pyridine / 3 h / 20 °C 3: 65 percent / AD-α-mix; CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 48 h / 4 °C 4: NaIO4 / tetrahydrofuran; H2O / 1 h / 20 °C 5: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 6: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 7: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |
-
-
935263-64-2
7-hydroxy-2-(4-methoxy-benzyloxy)-6,10,14,16a-tetramethyl-4,7,8,9,12,13,16,16a-octahydro-3aH-cyclopentacyclopentadecen-1-one

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 93 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C 2: (S,S)-N-[2-(2-PPh2-benzoylamino)cyclohexyl]-2-PPh2-benzamide / Pd2dba3*CHCl3 / CH2Cl2 / 20 °C 3: 1,2-dimethoxy-ethane / 1 h / 150 °C / microwave irradiation 4: Cs2CO3; Bu4NI / dimethylformamide / 2 h 5: 8.7 mg / pyridine / 3 h / 20 °C 6: 65 percent / AD-α-mix; CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 48 h / 4 °C 7: NaIO4 / tetrahydrofuran; H2O / 1 h / 20 °C 8: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 9: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 10: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |
-
-
935263-63-1
4-allyl-5-(10-hydroxy-3,7,11-trimethyl-dodeca-2,6,11-trienyl)-2-(4-methoxy-benzyloxy)-5-methyl-cyclopent-2-enone

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1: 44 percent / Grubbs second generation catalyst / benzene / 16 h / 20 °C 2: 93 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C 3: (S,S)-N-[2-(2-PPh2-benzoylamino)cyclohexyl]-2-PPh2-benzamide / Pd2dba3*CHCl3 / CH2Cl2 / 20 °C 4: 1,2-dimethoxy-ethane / 1 h / 150 °C / microwave irradiation 5: Cs2CO3; Bu4NI / dimethylformamide / 2 h 6: 8.7 mg / pyridine / 3 h / 20 °C 7: 65 percent / AD-α-mix; CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 48 h / 4 °C 8: NaIO4 / tetrahydrofuran; H2O / 1 h / 20 °C 9: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 10: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 11: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 2: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 3: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 8.7 mg / pyridine / 3 h / 20 °C 2: 65 percent / AD-α-mix; CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 48 h / 4 °C 3: NaIO4 / tetrahydrofuran; H2O / 1 h / 20 °C 4: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 5: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 6: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |
-
-
935263-73-3
4-allyl-5-(5-benzenesulfonyl-10-hydroxy-3,7,11-trimethyl-dodeca-2,6,11-trienyl)-2-(4-methoxy-benzyloxy)-5-methyl-cyclopent-2-enone

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1: 77 percent / NaBH4; DPPP / Pd(OAc)2 / dimethylsulfoxide 2: 44 percent / Grubbs second generation catalyst / benzene / 16 h / 20 °C 3: 93 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C 4: (S,S)-N-[2-(2-PPh2-benzoylamino)cyclohexyl]-2-PPh2-benzamide / Pd2dba3*CHCl3 / CH2Cl2 / 20 °C 5: 1,2-dimethoxy-ethane / 1 h / 150 °C / microwave irradiation 6: Cs2CO3; Bu4NI / dimethylformamide / 2 h 7: 8.7 mg / pyridine / 3 h / 20 °C 8: 65 percent / AD-α-mix; CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 48 h / 4 °C 9: NaIO4 / tetrahydrofuran; H2O / 1 h / 20 °C 10: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 11: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 12: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |
-
-
935263-67-5
acetic acid 2-(4-methoxy-benzyloxy)-6,10,14,16a-tetramethyl-3-(1-methyl-but-2-enyl)-1-oxo-1,3a,4,7,8,9,12,13,16,16a-decahydro-cyclopentacyclopentadecen-7-yl ester

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 65 percent / AD-α-mix; CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 48 h / 4 °C 2: NaIO4 / tetrahydrofuran; H2O / 1 h / 20 °C 3: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 4: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 5: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |
-
-
935263-69-7
acetic acid 3-(2-hydroxy-1-methyl-ethyl)-2-(4-methoxy-benzyloxy)-6,10,14,16a-tetramethyl-1-oxo-1,3a,4,7,8,9,12,13,16,16a-decahydro-cyclopentacyclopentadecen-7-yl ester

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 2: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |
-
-
935263-68-6
acetic acid 3-(2,3-dihydroxy-1-methyl-butyl)-2-(4-methoxy-benzyloxy)-6,10,14,16a-tetramethyl-1-oxo-1,3a,4,7,8,9,12,13,16,16a-decahydro-cyclopentacyclopentadecen-7-yl ester

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: NaIO4 / tetrahydrofuran; H2O / 1 h / 20 °C 2: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 3: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 4: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |
-
-
765-70-8
3-methyl-1,2-cyclopentanedione

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 19 steps 1.1: (R,R)-N-[2-(2-PPh2-benzoylamino)cyclohexyl]-2-PPh2-benzamide; Bu4NCl / Pd2dba3*CHCl3 / CH2Cl2 / -78 - 20 °C 1.2: 2,6-lutidine / CH2Cl2 / 16 h / -78 - 20 °C 2.1: CHCl3 / 0.5 h / 100 - 120 °C / microwave irradiation 2.2: Cs2CO3 / Pd(OAc)2 / acetonitrile / 0.5 h / 20 °C 3.1: MgBr2*Et2O / CH2Cl2 / 1 h / 20 °C 4.1: 79 percent / Cs2CO3; Bu4NI / dimethylformamide / 2.5 h / 20 °C 5.1: 84 percent / TBAF*3H2O / tetrahydrofuran / 2 h / 0 °C 6.1: 88 percent / PPh3; CBr4 / acetonitrile / 20 °C 7.1: 85 percent / LiHMDS; HMPA / tetrahydrofuran / 0.08 h / -40 °C 8.1: 77 percent / NaBH4; DPPP / Pd(OAc)2 / dimethylsulfoxide 9.1: 44 percent / Grubbs second generation catalyst / benzene / 16 h / 20 °C 10.1: 93 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C 11.1: (S,S)-N-[2-(2-PPh2-benzoylamino)cyclohexyl]-2-PPh2-benzamide / Pd2dba3*CHCl3 / CH2Cl2 / 20 °C 12.1: 1,2-dimethoxy-ethane / 1 h / 150 °C / microwave irradiation 13.1: Cs2CO3; Bu4NI / dimethylformamide / 2 h 14.1: 8.7 mg / pyridine / 3 h / 20 °C 15.1: 65 percent / AD-α-mix; CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 48 h / 4 °C 16.1: NaIO4 / tetrahydrofuran; H2O / 1 h / 20 °C 17.1: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 18.1: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 19.1: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |
-
-
95763-61-4
3,7-dimethyl-1-(phenylsulfonyl)-8-hydroxy-2(E),6(E)-octadiene

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 15 steps 1.1: 80 percent / L-diethyl tartrate; TBHP / titanium tetraisopropoxide / CH2Cl2; decane / 20 h / -20 °C 2.1: pyridine; iodine; PPh3 / acetonitrile; diethyl ether / 2 h / 0 °C 2.2: 74 percent / water / acetonitrile; diethyl ether / 8 h / 38 °C 3.1: 85 percent / LiHMDS; HMPA / tetrahydrofuran / 0.08 h / -40 °C 4.1: 77 percent / NaBH4; DPPP / Pd(OAc)2 / dimethylsulfoxide 5.1: 44 percent / Grubbs second generation catalyst / benzene / 16 h / 20 °C 6.1: 93 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C 7.1: (S,S)-N-[2-(2-PPh2-benzoylamino)cyclohexyl]-2-PPh2-benzamide / Pd2dba3*CHCl3 / CH2Cl2 / 20 °C 8.1: 1,2-dimethoxy-ethane / 1 h / 150 °C / microwave irradiation 9.1: Cs2CO3; Bu4NI / dimethylformamide / 2 h 10.1: 8.7 mg / pyridine / 3 h / 20 °C 11.1: 65 percent / AD-α-mix; CH3SO2NH2 / 2-methyl-propan-2-ol; H2O / 48 h / 4 °C 12.1: NaIO4 / tetrahydrofuran; H2O / 1 h / 20 °C 13.1: 6.5 mg / NaBH4 / CH2Cl2; methanol / 0.5 h / -78 °C 14.1: 89 percent / aq. LiOH / tetrahydrofuran; methanol / 2 h / 20 °C 15.1: 74 percent / dimethyl sulfide; MgBr2*Et2O / CH2Cl2 / 0.67 h / 20 °C View Scheme |
-
-
87920-55-6
10-hydroxy-4,8-dimethyldeca-4,8-dienal

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: 76 percent / 2,6-lutidine / CH2Cl2 / 2 h / -78 °C 2.1: Ni(cod)2; (R)-P-ferrocenyl-P-(p-xylyl)phenylphosphine; triethylborane / ethyl acetate / 16 h / 0 °C 2.2: 2,6-lutidine / CH2Cl2 / 2 h / -78 °C 2.3: 1.05 g / NaOH / methanol / 0.17 h / 0 °C 3.1: 89 percent / N-methylmorpholine N-oxide; TPAP / CH2Cl2 / 2.5 h / 0 °C 4.1: 84 percent / aq. HCl / tetrahydrofuran / 8 h 5.1: triphenylphosphine; iodine; imidazole / benzene; diethyl ether / 0.92 h 6.1: LiHMDS / tetrahydrofuran / 1.5 h / 0 °C 7.1: 62 percent / NaH; H2O / toluene / 15 h 8.1: 72 percent / TBAF / tetrahydrofuran / 3 h 9.1: potassium bis(trimethylsilyl)amide / tetrahydrofuran / 1 h / -78 °C 9.2: triethylphosphite; oxygen / tetrahydrofuran / 0.75 h 10.1: 2.5 mg / potassium carbonate / methanol / 2 h / 20 °C View Scheme |
-
-
130790-62-4
(R,2E,6E)-10,11-dihydroxy-3,7,11-trimethyldodeca-2,6-dienyl acetate

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1.1: potassium carbonate / methanol / 1 h / 0 - 20 °C 2.1: 1.34 g / aq. sodium periodinate / methanol / 0 °C 3.1: 76 percent / 2,6-lutidine / CH2Cl2 / 2 h / -78 °C 4.1: Ni(cod)2; (R)-P-ferrocenyl-P-(p-xylyl)phenylphosphine; triethylborane / ethyl acetate / 16 h / 0 °C 4.2: 2,6-lutidine / CH2Cl2 / 2 h / -78 °C 4.3: 1.05 g / NaOH / methanol / 0.17 h / 0 °C 5.1: 89 percent / N-methylmorpholine N-oxide; TPAP / CH2Cl2 / 2.5 h / 0 °C 6.1: 84 percent / aq. HCl / tetrahydrofuran / 8 h 7.1: triphenylphosphine; iodine; imidazole / benzene; diethyl ether / 0.92 h 8.1: LiHMDS / tetrahydrofuran / 1.5 h / 0 °C 9.1: 62 percent / NaH; H2O / toluene / 15 h 10.1: 72 percent / TBAF / tetrahydrofuran / 3 h 11.1: potassium bis(trimethylsilyl)amide / tetrahydrofuran / 1 h / -78 °C 11.2: triethylphosphite; oxygen / tetrahydrofuran / 0.75 h 12.1: 2.5 mg / potassium carbonate / methanol / 2 h / 20 °C View Scheme |
-
-
147458-56-8
(4E,8E)-10-(tert-butyldimethylsilyloxy)-4,8-dimethyldeca-4,8-dienal

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: Ni(cod)2; (R)-P-ferrocenyl-P-(p-xylyl)phenylphosphine; triethylborane / ethyl acetate / 16 h / 0 °C 1.2: 2,6-lutidine / CH2Cl2 / 2 h / -78 °C 1.3: 1.05 g / NaOH / methanol / 0.17 h / 0 °C 2.1: 89 percent / N-methylmorpholine N-oxide; TPAP / CH2Cl2 / 2.5 h / 0 °C 3.1: 84 percent / aq. HCl / tetrahydrofuran / 8 h 4.1: triphenylphosphine; iodine; imidazole / benzene; diethyl ether / 0.92 h 5.1: LiHMDS / tetrahydrofuran / 1.5 h / 0 °C 6.1: 62 percent / NaH; H2O / toluene / 15 h 7.1: 72 percent / TBAF / tetrahydrofuran / 3 h 8.1: potassium bis(trimethylsilyl)amide / tetrahydrofuran / 1 h / -78 °C 8.2: triethylphosphite; oxygen / tetrahydrofuran / 0.75 h 9.1: 2.5 mg / potassium carbonate / methanol / 2 h / 20 °C View Scheme |
-
-
615584-43-5
3-methyl-5-trimethylsilanyl-2,3,3a,6a-tetrahydrocyclopenta[b]furan-6-one

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1.1: tert-butyllithium / diethyl ether; tetrahydrofuran; hexane / 0.5 h / -78 - 20 °C 1.2: CuI; dimethyl sulfide / hexane; tetrahydrofuran; diethyl ether 1.3: 54 percent / hexane; tetrahydrofuran; diethyl ether / 6 h / -40 °C 2.1: sodium borohydride / methanol / 3 h / 0 °C 3.1: 1.69 g / TBAF / tetrahydrofuran / 16 h / -78 - 20 °C 4.1: 94 percent / potassium tert-butoxide / dimethylsulfoxide / 0.25 h 5.1: 86 percent / triethylamine / tetrahydrofuran / 1 h / 20 °C 6.1: Ni(cod)2; (R)-P-ferrocenyl-P-(p-xylyl)phenylphosphine; triethylborane / ethyl acetate / 16 h / 0 °C 6.2: 2,6-lutidine / CH2Cl2 / 2 h / -78 °C 6.3: 1.05 g / NaOH / methanol / 0.17 h / 0 °C 7.1: 89 percent / N-methylmorpholine N-oxide; TPAP / CH2Cl2 / 2.5 h / 0 °C 8.1: 84 percent / aq. HCl / tetrahydrofuran / 8 h 9.1: triphenylphosphine; iodine; imidazole / benzene; diethyl ether / 0.92 h 10.1: LiHMDS / tetrahydrofuran / 1.5 h / 0 °C 11.1: 62 percent / NaH; H2O / toluene / 15 h 12.1: 72 percent / TBAF / tetrahydrofuran / 3 h 13.1: potassium bis(trimethylsilyl)amide / tetrahydrofuran / 1 h / -78 °C 13.2: triethylphosphite; oxygen / tetrahydrofuran / 0.75 h 14.1: 2.5 mg / potassium carbonate / methanol / 2 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at -78℃; | 95% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| In methanol; diethyl ether Ambient temperature; | 65 mg |
-
-
67-56-1
methanol

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol | 17 mg |
-
-
76-83-5
trityl chloride

-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| With bisacodyl In dichloromethane at 37℃; for 120h; | 29 mg |
-
-
146436-22-8
(−)-terpestacin

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In dichloromethane | |
| With magnesium sulfate; toluene-4-sulfonic acid In dichloromethane Ambient temperature; | 60 mg |
-
-
146436-22-8
(−)-terpestacin


| Conditions | Yield |
|---|---|
| With deuteromethanol; water-d2; magnesium sulfate; toluene-4-sulfonic acid In dichloromethane Ambient temperature; Yield given. Yields of byproduct given; |
Related products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View