Ality Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Dayang Chem (Hangzhou) Co.,Ltd.
As a leading manufacturer and supplier of chemicals in China, DayangChem not only supply popular chemicals, but also DayangChem’s R&D center offer custom synthesis according to the contract research and development services for the fine chemicals, ph
Shandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:25999-31-9
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHebei Nengqian Chemical Import and Export Co., LTD
Our advantages: 1. All inquiries will be replied within 12 hours. 2. Dedication to quality, supply & service. 3. Strictly on selecting raw materials. 4. Reasonable & competitive price, fast lead time. 5. Sample is available for your eva
Henan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:25999-31-9
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryAfine Chemicals Limited
Our Services 1. New Molecules R&D 2. Own test center HPLC NMR GC LC-MS 3. API and Intermediates from China reputed manufacturers 4. Documents support COA MOA MSDS DMF open part Our advantages 1. Government awarded company. Top 100 enter
Cas:25999-31-9
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquirySHANGHAI T&W PHARMACEUTICAL CO., LTD.
A substitute for perfluorooctanoic acid, mainly used as a surfactant, dispersant, additive, etc Appearance:White solid or Colorless liquid Purity:99.3 % We will ship the goods in a timely manner as required We can provide relevant documents acc
Xiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
TAIZHOU ZHENYU BIOTECHNOLOGY CO., LTD
Zhenyu biotech exported this product to many countries and regions at best price. if you are looking for the material's manufacturer or supplier in china, zhenyu biotech is your best choice. pls contact with us freely for getting detailed
KAISA GROUP INC
1.Applied in food field.it can improve the immune system and prolong life. 2.Appliedin cosmetic field.it can improve the skin care. 3.Applied in pharmaceutical field.it can treat various dieases. 4.Our product quality assurance will make our customer
Cas:25999-31-9
Min.Order:1 Metric Ton
FOB Price: $7.0 / 8.0
Type:Trading Company
inquiryHunan chemfish Pharmaceutical co.,Ltd
Appearance:95%+ Package:R&D,Pilot run Transportation:per client require Port:Express ,Air, Sea
Hangzhou J&H Chemical Co., Ltd.
J&H CHEM is one of China's leading providers of integrated fine chemical services including offering, research and development, Custom manufacturing business, as well as other Value-added customer services, for diversified range products of chemicals
Henan Tianfu Chemical Co., Ltd.
1.Our services:A.Supply sampleB.The packing also can be according the customers` requirmentC.Any inquiries will be replied within 24 hoursD.we provide Commerical Invoice, Packing List, Bill of loading, COA , Health certificate and Origin certificate.
Cas:25999-31-9
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryAntimex Chemical Limied
Ansciep Chemical is a professional enterprise manufacturing and distributing fine chemicals and speciality chemicals. We have been dedicated to heterocycle compounds and phenyl rings for tens of years. This is our mature product for export. Our quali
Cas:25999-31-9
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHunan Russell Chemicals Technology Co.,Ltd
high purity lowest priceAppearance:solid or liquid Storage:in sealed air resistant place Package:Foil bag; Drum; Plastic bottle Application:Pharma;Industry;Agricultural Transportation:by sea or air Port:any port in China
Guangdong Juda Chemical Industrial Co.,Limited
Appearance:solid or liquid Storage:sealed in cool and dry place Package:As customer's requested Application:Pharma Intermediate Transportation:by courier/air/sea Port:Any port in China
Changchun Artel lmport and Export trade company
Supply top quality products with a reasonable price Application:api
Henan Kanbei Chemical Co.,LTD
factory?direct?saleAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:healing drugs Transportation:By sea Port:Shanghai/tianjin
Asure Biochem CO.,LTD.
*stable and better quality products*efficient and meticulous servicesAppearance:Powder Storage:Store in dry, cool and ventilated place Package:1kg/tin 5kg/tin 25kg/carton Application:Pharmaceutical raw materials, making injection powder oral agent Tr
BOC Sciences
BOC Sciences provides a wide range of research chemicals and biochemicals including inhibitors, building blocks, GMP Products, impurities and metabolites, APIs for Veterinary, Natural Compounds, ADCs, Stem Cell Molecule and chiral compounds.Appearanc
Shanghai Chinqesen Biotechnology Co., Ltd.
Good Quality Package:1kg/bag Application:Medical or chemical Transportation:Air/Train/Sea Port:Shenzhen
ZHEJIANG JIUZHOU CHEM CO.,LTD
factory?direct?saleAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:healing drugs Transportation:By sea Port:Shanghai/tianjin
Amadis Chemical Co., Ltd.
1.Professional synthesis laboratory and production base. 2.Strong synthesis team and service team. 3.Professional data management system. 4.We provide the professional test date and product information ,ex. HNMR ,CNMR,FNMR, HPLC/G
Zhengzhou Kingorgchem Chemical Technology Co., Ltd.
1. Subsidiary of Institute of Chemistry, Henan Academy of Sciences, national research platform;2. About 30 years’ experience in this field since 1983;3. An experienced R&D team consisting of Doctors and Masters;4. Various types of analytical instrume
Cas:25999-31-9
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryJilin haofei import and export trade Co.,Ltd
Price, service, company and transport advantage: 1. Best service, place of origin China, high quality, and reasonable price. 2. It's customers' right to choose the package (EMS, DHL, FEDEX, UPS). 3. It's customers' right
Shanghai united Scientific Co.,Ltd.
United Scientific Company Located in Shanghai of China , is a competitive player in the global specialty and fine chemical market. Fenghua has both the expertise and flexibility to produce a wide range of chemicals. Focusing on developing the innovat
Cas:25999-31-9
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryPure Chemistry Scientific Inc.
Lasalocid Application:829907
Zhejiang Huida Biotech Co., Ltd
High quality Short lead time Cost competitiveness Appearance:Solid powder Storage:Store in a tight container at -20℃. Package:As per request Application:Anti-parasitic Ionophore antibiotic isolated from certain Streptomyces sp Transportation:by ai
Nanjing Raymon Biotech Co., Ltd.
Lasalocid Storage:keep in dry and cool condition Package:25kg or according to cutomer's demand Application:Chemical research/pharma intermediate Transportation:By Sea,by Air,By courier like DHL or Fedx. Port:Shanghai/Shenzhen
Topbatt Chemical Co., Ltd.
Topbatt Chemical Co., Ltd., Established in 2019, located in Shenzhen, Guangdong Province, is a Manufacturer and Trading company which specialized in fine chemicals like Pharmaceutical Reference Standards and Stable Isotopes. Our Stable Isotopes produ
Synthetic route
-
-
41733-86-2
lasalocid benzyl ester

-
-
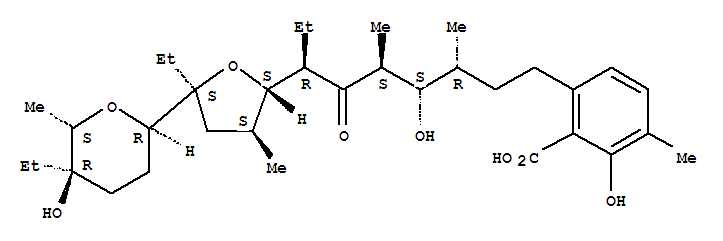
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| With hydrogen; palladium dihydroxide In ethanol Ambient temperature; Yield given; | |
| With hydrogen; palladium on activated charcoal In ethanol for 3h; Ambient temperature; |
-
-
67045-68-5
3-methyl-6-(3(R)-formylbutyl)salicylic acid benzyl ester

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 2: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
25999-20-6
lasalocid sodium

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dioxane 2: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme | |
| With sulfuric acid; water In dichloromethane pH=1.5; | |
| With sulfuric acid In dichloromethane; water pH=1.5; | |
| With sulfuric acid In dichloromethane; water | 0.75 g |
-
-
73657-53-1
(2S)-2-<(2S,3S,5S)-5-ethyl-5-<(2R,5R,6S)-5-ethyl-5-hydroxy-6-methyltetrahydropyran-2-yl>-3-methyltetrahydrofur-2-yl>butan-1-ol

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 78 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 2: 89 percent / tetrahydrofuran / 0.5 h / 0 °C 3: 90 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 4: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 5: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme | |
| Multi-step reaction with 5 steps 1: 84 percent / PCC, 3A-MS / CH2Cl2 / Ambient temperature 2: tetrahydrofuran / 0 °C 3: PCC, 3A-MS / CH2Cl2 / Ambient temperature 5: H2 / Pd(OH)2 / ethanol / Ambient temperature View Scheme |
-
-
84911-92-2, 84985-23-9, 87678-43-1, 87678-51-1
(3RS,4S)-4-<(2S,3S,5S)-5-ethyl-5-<(2R,5R,6S)-5-ethyl-5-hydroxy-6-methyltetrahydropyran-2-yl>-3-methyltetrahydrofur-2-yl>hexan-3-ol

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 90 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 2: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 3: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme | |
| Multi-step reaction with 3 steps 1: PCC, 3A-MS / CH2Cl2 / Ambient temperature 3: H2 / Pd(OH)2 / ethanol / Ambient temperature View Scheme |
-
-
84911-91-1
(2R)-2-<(2S,3S,5S)-5-ethyl-5-<(2R,5R,6S)-5-ethyl-5-hydroxy-6-methyltetrahydropyran-2-yl>-3-methyltetrahydrofur-2-yl>butanal

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 89 percent / tetrahydrofuran / 0.5 h / 0 °C 2: 90 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 3: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 4: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme | |
| Multi-step reaction with 4 steps 1: tetrahydrofuran / 0 °C 2: PCC, 3A-MS / CH2Cl2 / Ambient temperature 4: H2 / Pd(OH)2 / ethanol / Ambient temperature View Scheme |
-
-
31478-26-9, 87678-18-0
4(R)-<5(S)-ethyl-3(S)-methyl-5-(5(R)-ethyl-5-hydroxy-6(S)-methyl-2(R)-tetrahydropyranyl)-2(S)-tetrahydrofuryl>hexan-3-one

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 2: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 2: H2 / Pd(OH)2 / ethanol / Ambient temperature View Scheme |
-
-
75371-81-2, 84911-50-2, 84911-98-8
3-methyl-6-(3(R)-methyl-4,5-O-isopropylidenepentyl)salicylic acid methyl ester

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: KH / tetrahydrofuran / 1.) 0 deg C, 20 min, 2.) 0 deg C, 1 h, 3.) room temperature, 0.5 h 2: 1.) BuLi, n-propanethiol / 1.) pentane, hexane, 0 deg C, 15 min, 2.) HMPA, 0 deg C, 10 min, room temperature, 45 min, 3.) room temperature, 2 h 3: 10percent HCl / H2O; tetrahydrofuran / 1.) room temperature, 4 h, 2.) 50 deg C, 7 h 4: 80 percent / NaIO4 / methanol; H2O / 2 h / Ambient temperature 5: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 6: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
73657-51-9
benzyl 2(S)-<5(S)-ethyl-3(S)-methyl-5-(5(R)-ethyl-5-hydroxy-6(S)-methyl-2(R)-tetrahydropyranyl)-2(S)-tetrahydrofuryl>butyl ether

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 94 percent / oxalyl chloride, dimethyl sulfoxide, Et3N / CH2Cl2 / 1.) -60 deg C, 10 min, 2.) 15 min, 3.) warm to room temperature 2: 94 percent / n-BuLi / tetrahydrofuran; hexane / 1.) room temperature, 1 h, 2.) -78 deg C to room temperature, 10 h 3: NaHCO3, MCPBA / CH2Cl2 / 3 h / Ambient temperature 4: 90 percent / CuI / pentane; diethyl ether / 1.) 0 deg C, 15 min, 2.) 3 h 5: 98 percent / Li, NH3 / tetrahydrofuran / 1 h / Heating 6: 78 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 7: 89 percent / tetrahydrofuran / 0.5 h / 0 °C 8: 90 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 9: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 10: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
73657-52-0
benzyl 2(S)-<5(S)-ethyl-3(S)-methyl-5-(3(R)-1,5-dioxo-4(S)-methylspiro<2.5>-6(R)-octyl)-2(S)-tetrahydrofuryl>butyl ether

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 90 percent / CuI / pentane; diethyl ether / 1.) 0 deg C, 15 min, 2.) 3 h 2: 98 percent / Li, NH3 / tetrahydrofuran / 1 h / Heating 3: 78 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 4: 89 percent / tetrahydrofuran / 0.5 h / 0 °C 5: 90 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 6: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 7: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
75371-80-1
2-(dibenzylamino)-3-methyl-6-(3(R)-methyl-4-pentenyl)benzoic acid methyl ester

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 91 percent / 4-methylmorpholine 4-oxide, OsO4 / H2O; 2-methyl-propan-2-ol / 24 h / Ambient temperature 2: 93 percent / H2 / Pd/C / ethanol / 10 h / 2585.7 Torr 3: 85 percent / HBF4, isoamyl nitrite / ethanol / 0.5 h / 0 °C 4: 98 percent / p-toluenesulfonic acid / acetone / 0.25 h / Ambient temperature 5: KH / tetrahydrofuran / 1.) 0 deg C, 20 min, 2.) 0 deg C, 1 h, 3.) room temperature, 0.5 h 6: 1.) BuLi, n-propanethiol / 1.) pentane, hexane, 0 deg C, 15 min, 2.) HMPA, 0 deg C, 10 min, room temperature, 45 min, 3.) room temperature, 2 h 7: 10percent HCl / H2O; tetrahydrofuran / 1.) room temperature, 4 h, 2.) 50 deg C, 7 h 8: 80 percent / NaIO4 / methanol; H2O / 2 h / Ambient temperature 9: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 10: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
84985-06-8
benzyl 2(S)-<5(S)-carbomethoxy-3(S)-methyl-5-(5,6-dihydro)-5(S)-(methoxymethylenoxy)-6(S)-methyl-2(R)-pyranyl-2(S)-tetrahydrofuryl>butyl ether

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 15 steps 1: 85 percent / H2 / Ni(Ra) / ethyl acetate / 3 h / Ambient temperature 2: 95 percent / DIBAL / diethyl ether; hexane / 1 h / -78 °C 3: 88 percent / n-BuLi / tetrahydrofuran; hexane / 1.) room temperature, 1 h, 2.) -78 deg C to room temperature, 10 h 4: 90 percent / H2 / Ni(Ra) / ethyl acetate / 3 h / Ambient temperature 5: 100 percent / 10percent HCl / tetrahydrofuran; H2O / 16 h / 50 °C 6: 94 percent / oxalyl chloride, dimethyl sulfoxide, Et3N / CH2Cl2 / 1.) -60 deg C, 10 min, 2.) 15 min, 3.) warm to room temperature 7: 94 percent / n-BuLi / tetrahydrofuran; hexane / 1.) room temperature, 1 h, 2.) -78 deg C to room temperature, 10 h 8: NaHCO3, MCPBA / CH2Cl2 / 3 h / Ambient temperature 9: 90 percent / CuI / pentane; diethyl ether / 1.) 0 deg C, 15 min, 2.) 3 h 10: 98 percent / Li, NH3 / tetrahydrofuran / 1 h / Heating 11: 78 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 12: 89 percent / tetrahydrofuran / 0.5 h / 0 °C 13: 90 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 14: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 15: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
75371-88-9, 84911-49-9, 84911-97-7
3-methyl-6-(3(R)-methyl-4,5-dihydroxypentyl)salicylic acid methyl ester

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 98 percent / p-toluenesulfonic acid / acetone / 0.25 h / Ambient temperature 2: KH / tetrahydrofuran / 1.) 0 deg C, 20 min, 2.) 0 deg C, 1 h, 3.) room temperature, 0.5 h 3: 1.) BuLi, n-propanethiol / 1.) pentane, hexane, 0 deg C, 15 min, 2.) HMPA, 0 deg C, 10 min, room temperature, 45 min, 3.) room temperature, 2 h 4: 10percent HCl / H2O; tetrahydrofuran / 1.) room temperature, 4 h, 2.) 50 deg C, 7 h 5: 80 percent / NaIO4 / methanol; H2O / 2 h / Ambient temperature 6: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 7: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
84911-36-4
2(S)-<5(S)-ethyl-3(S)-methyl-5-(5(R)-ethyl-5-(trimethylsiloxy)-6(S)-methyl-2(R)-tetrahydropyranyl)-2(S)-tetrahydrofuryl>butan-1-ol

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 92 percent / n-Bu4NF / tetrahydrofuran / 4 h 2: 78 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 3: 89 percent / tetrahydrofuran / 0.5 h / 0 °C 4: 90 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 5: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 6: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
75371-87-8, 84911-48-8, 84911-96-6
2-amino-3-methyl-6-(3(R)-methyl-4,5-O-isopropylidenepentyl)benzoic acid methyl ester

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 85 percent / HBF4, isoamyl nitrite / ethanol / 0.5 h / 0 °C 2: 98 percent / p-toluenesulfonic acid / acetone / 0.25 h / Ambient temperature 3: KH / tetrahydrofuran / 1.) 0 deg C, 20 min, 2.) 0 deg C, 1 h, 3.) room temperature, 0.5 h 4: 1.) BuLi, n-propanethiol / 1.) pentane, hexane, 0 deg C, 15 min, 2.) HMPA, 0 deg C, 10 min, room temperature, 45 min, 3.) room temperature, 2 h 5: 10percent HCl / H2O; tetrahydrofuran / 1.) room temperature, 4 h, 2.) 50 deg C, 7 h 6: 80 percent / NaIO4 / methanol; H2O / 2 h / Ambient temperature 7: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 8: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
84911-38-6
(2S,6R)-6-[(2S,4S,5S)-5-((S)-1-Benzyloxymethyl-propyl)-2-ethyl-4-methyl-tetrahydro-furan-2-yl]-3-eth-(Z)-ylidene-2-methyl-tetrahydro-pyran

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1: 1.) O3, 2.) NaBH4 / 1.) CH3OH, CH2Cl2, -78 deg C, 2.) room temperature, 10 h 2: 94 percent / oxalyl chloride, dimethyl sulfoxide, Et3N / CH2Cl2 / 1.) -60 deg C, 10 min, 2.) 15 min, 3.) warm to room temperature 3: 94 percent / n-BuLi / tetrahydrofuran; hexane / 1.) room temperature, 1 h, 2.) -78 deg C to room temperature, 10 h 4: NaHCO3, MCPBA / CH2Cl2 / 3 h / Ambient temperature 5: 90 percent / CuI / pentane; diethyl ether / 1.) 0 deg C, 15 min, 2.) 3 h 6: 98 percent / Li, NH3 / tetrahydrofuran / 1 h / Heating 7: 78 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 8: 89 percent / tetrahydrofuran / 0.5 h / 0 °C 9: 90 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 10: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 11: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: diethyl ether 2: 99 percent / LiAlH4 / diethyl ether / 1 h 3: 92 percent / n-Bu4NF / tetrahydrofuran / 4 h 4: 78 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 5: 89 percent / tetrahydrofuran / 0.5 h / 0 °C 6: 90 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 7: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 8: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
84911-35-3
methyl 2(R)-<5(S)-ethyl-3(S)-methyl-5-(5(R)-ethyl-5-(trimethylsiloxy)-6(S)-methyl-2(R)-tetrahydropyranyl)-2(S)-tetrahydrofuryl>butanoate

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 99 percent / LiAlH4 / diethyl ether / 1 h 2: 92 percent / n-Bu4NF / tetrahydrofuran / 4 h 3: 78 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 4: 89 percent / tetrahydrofuran / 0.5 h / 0 °C 5: 90 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 6: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 7: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
75371-89-0, 84911-51-3, 84911-99-9
2-(β-methoxyethoxymethyl)-3-methyl-6-(3(R)-methyl-4,5-O-isopropylidenepentyl)salicylic acid methyl ester

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 1.) BuLi, n-propanethiol / 1.) pentane, hexane, 0 deg C, 15 min, 2.) HMPA, 0 deg C, 10 min, room temperature, 45 min, 3.) room temperature, 2 h 2: 10percent HCl / H2O; tetrahydrofuran / 1.) room temperature, 4 h, 2.) 50 deg C, 7 h 3: 80 percent / NaIO4 / methanol; H2O / 2 h / Ambient temperature 4: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 5: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
84911-52-4, 84912-00-5
2-(β-methoxyethoxymethyl)-3-methyl-6-(3(R)-methyl-4,5-O-isopropylidenepentyl)salicylic acid benzyl ester

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 10percent HCl / H2O; tetrahydrofuran / 1.) room temperature, 4 h, 2.) 50 deg C, 7 h 2: 80 percent / NaIO4 / methanol; H2O / 2 h / Ambient temperature 3: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 4: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
75371-86-7, 84911-47-7, 84911-95-5
2-(dibenzylamino)-3-methyl-6-(3(R)-methyl-4,5-O-isopropylidenepentyl)benzoic acid methyl ester

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 93 percent / H2 / Pd/C / ethanol / 10 h / 2585.7 Torr 2: 85 percent / HBF4, isoamyl nitrite / ethanol / 0.5 h / 0 °C 3: 98 percent / p-toluenesulfonic acid / acetone / 0.25 h / Ambient temperature 4: KH / tetrahydrofuran / 1.) 0 deg C, 20 min, 2.) 0 deg C, 1 h, 3.) room temperature, 0.5 h 5: 1.) BuLi, n-propanethiol / 1.) pentane, hexane, 0 deg C, 15 min, 2.) HMPA, 0 deg C, 10 min, room temperature, 45 min, 3.) room temperature, 2 h 6: 10percent HCl / H2O; tetrahydrofuran / 1.) room temperature, 4 h, 2.) 50 deg C, 7 h 7: 80 percent / NaIO4 / methanol; H2O / 2 h / Ambient temperature 8: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 9: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
84911-90-0
benzyl 2(S)-<5(S)-ethyl-3(S)-methyl-5-(6(S)-methyl-5-methylene-2(R)-tetrahydropyranyl)-2(S)-tetrahydrofuryl>butyl ether

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: NaHCO3, MCPBA / CH2Cl2 / 3 h / Ambient temperature 2: 90 percent / CuI / pentane; diethyl ether / 1.) 0 deg C, 15 min, 2.) 3 h 3: 98 percent / Li, NH3 / tetrahydrofuran / 1 h / Heating 4: 78 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 5: 89 percent / tetrahydrofuran / 0.5 h / 0 °C 6: 90 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 7: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 8: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
84911-37-5
benzyl 2(S)-<5(S)-ethyl-3(S)-methyl-5-(5(R)-ethyl-5-hydroxy-6(S)-methyl-2(R)-tetrahydropyranyl)-2(S)-tetrahydrofuryl>butyl ether

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 98 percent / Li, NH3 / tetrahydrofuran / 1 h / Heating 2: 78 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 3: 89 percent / tetrahydrofuran / 0.5 h / 0 °C 4: 90 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 5: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 6: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme | |
| Multi-step reaction with 12 steps 1: KH, CS2, CH3I / tetrahydrofuran / 1.) 5 h, 2.) 30 min 2: 1.) O3, 2.) NaBH4 / 1.) CH3OH, CH2Cl2, -78 deg C, 2.) room temperature, 10 h 3: 94 percent / oxalyl chloride, dimethyl sulfoxide, Et3N / CH2Cl2 / 1.) -60 deg C, 10 min, 2.) 15 min, 3.) warm to room temperature 4: 94 percent / n-BuLi / tetrahydrofuran; hexane / 1.) room temperature, 1 h, 2.) -78 deg C to room temperature, 10 h 5: NaHCO3, MCPBA / CH2Cl2 / 3 h / Ambient temperature 6: 90 percent / CuI / pentane; diethyl ether / 1.) 0 deg C, 15 min, 2.) 3 h 7: 98 percent / Li, NH3 / tetrahydrofuran / 1 h / Heating 8: 78 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 9: 89 percent / tetrahydrofuran / 0.5 h / 0 °C 10: 90 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 11: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 12: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
84911-89-7, 87678-36-2
benzyl 2(S)-<5(S)-ethyl-3(S)-methyl-5-(6(S)-methyl-5-oxo-2(R)-tetrahydropyranyl)-2(S)-tetrahydrofuryl>butyl ether

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 94 percent / n-BuLi / tetrahydrofuran; hexane / 1.) room temperature, 1 h, 2.) -78 deg C to room temperature, 10 h 2: NaHCO3, MCPBA / CH2Cl2 / 3 h / Ambient temperature 3: 90 percent / CuI / pentane; diethyl ether / 1.) 0 deg C, 15 min, 2.) 3 h 4: 98 percent / Li, NH3 / tetrahydrofuran / 1 h / Heating 5: 78 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 6: 89 percent / tetrahydrofuran / 0.5 h / 0 °C 7: 90 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 8: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 9: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
75371-90-3, 84911-53-5, 84912-01-6, 87598-86-5, 87599-04-0
3-methyl-6-(3(R)-methyl-4,5-dihydroxypentyl)salicylic acid benzyl ester

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 80 percent / NaIO4 / methanol; H2O / 2 h / Ambient temperature 2: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 3: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
84985-18-2
benzyl 2(S)-<5(S)-ethyl-3(S)-methyl-5-(5(S)-(methoxymethylenoxy)-6(S)-methyl-2(R)-tetrahydropyranyl)-2(S)-tetrahydrofuryl>butyl ether

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1: 100 percent / 10percent HCl / tetrahydrofuran; H2O / 16 h / 50 °C 2: 94 percent / oxalyl chloride, dimethyl sulfoxide, Et3N / CH2Cl2 / 1.) -60 deg C, 10 min, 2.) 15 min, 3.) warm to room temperature 3: 94 percent / n-BuLi / tetrahydrofuran; hexane / 1.) room temperature, 1 h, 2.) -78 deg C to room temperature, 10 h 4: NaHCO3, MCPBA / CH2Cl2 / 3 h / Ambient temperature 5: 90 percent / CuI / pentane; diethyl ether / 1.) 0 deg C, 15 min, 2.) 3 h 6: 98 percent / Li, NH3 / tetrahydrofuran / 1 h / Heating 7: 78 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 8: 89 percent / tetrahydrofuran / 0.5 h / 0 °C 9: 90 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 10: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 11: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
84911-87-5, 84985-13-7, 84985-14-8, 84985-15-9, 87678-33-9
benzyl 2(S)-<5(R)-vinyl-3(S)-methyl-5-(5(S)-(methoxymethylenoxy)-6(S)-methyl-2(R)-tetrahydropyranyl)-2(S)-tetrahydrofuryl>butyl ether

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1: 90 percent / H2 / Ni(Ra) / ethyl acetate / 3 h / Ambient temperature 2: 100 percent / 10percent HCl / tetrahydrofuran; H2O / 16 h / 50 °C 3: 94 percent / oxalyl chloride, dimethyl sulfoxide, Et3N / CH2Cl2 / 1.) -60 deg C, 10 min, 2.) 15 min, 3.) warm to room temperature 4: 94 percent / n-BuLi / tetrahydrofuran; hexane / 1.) room temperature, 1 h, 2.) -78 deg C to room temperature, 10 h 5: NaHCO3, MCPBA / CH2Cl2 / 3 h / Ambient temperature 6: 90 percent / CuI / pentane; diethyl ether / 1.) 0 deg C, 15 min, 2.) 3 h 7: 98 percent / Li, NH3 / tetrahydrofuran / 1 h / Heating 8: 78 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 9: 89 percent / tetrahydrofuran / 0.5 h / 0 °C 10: 90 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 11: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 12: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
84985-12-6
benzyl 2(S)-<5(S)-formyl-3(S)-methyl-5-(5(S)-(methoxymethylenoxy)-6(S)-methyl-2(R)-tetrahydropyranyl)-2(S)-tetrahydrofuryl>butyl ether

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1: 88 percent / n-BuLi / tetrahydrofuran; hexane / 1.) room temperature, 1 h, 2.) -78 deg C to room temperature, 10 h 2: 90 percent / H2 / Ni(Ra) / ethyl acetate / 3 h / Ambient temperature 3: 100 percent / 10percent HCl / tetrahydrofuran; H2O / 16 h / 50 °C 4: 94 percent / oxalyl chloride, dimethyl sulfoxide, Et3N / CH2Cl2 / 1.) -60 deg C, 10 min, 2.) 15 min, 3.) warm to room temperature 5: 94 percent / n-BuLi / tetrahydrofuran; hexane / 1.) room temperature, 1 h, 2.) -78 deg C to room temperature, 10 h 6: NaHCO3, MCPBA / CH2Cl2 / 3 h / Ambient temperature 7: 90 percent / CuI / pentane; diethyl ether / 1.) 0 deg C, 15 min, 2.) 3 h 8: 98 percent / Li, NH3 / tetrahydrofuran / 1 h / Heating 9: 78 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 10: 89 percent / tetrahydrofuran / 0.5 h / 0 °C 11: 90 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 12: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 13: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
84985-09-1
benzyl 2(S)-<5(S)-carbomethoxy-3(S)-methyl-5-(5(S)-(methoxymethylenoxy)-6(S)-methyl-2(R)-tetrahydropyranyl)-2(S)-tetrahydrofuryl>butyl ether

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1: 95 percent / DIBAL / diethyl ether; hexane / 1 h / -78 °C 2: 88 percent / n-BuLi / tetrahydrofuran; hexane / 1.) room temperature, 1 h, 2.) -78 deg C to room temperature, 10 h 3: 90 percent / H2 / Ni(Ra) / ethyl acetate / 3 h / Ambient temperature 4: 100 percent / 10percent HCl / tetrahydrofuran; H2O / 16 h / 50 °C 5: 94 percent / oxalyl chloride, dimethyl sulfoxide, Et3N / CH2Cl2 / 1.) -60 deg C, 10 min, 2.) 15 min, 3.) warm to room temperature 6: 94 percent / n-BuLi / tetrahydrofuran; hexane / 1.) room temperature, 1 h, 2.) -78 deg C to room temperature, 10 h 7: NaHCO3, MCPBA / CH2Cl2 / 3 h / Ambient temperature 8: 90 percent / CuI / pentane; diethyl ether / 1.) 0 deg C, 15 min, 2.) 3 h 9: 98 percent / Li, NH3 / tetrahydrofuran / 1 h / Heating 10: 78 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 11: 89 percent / tetrahydrofuran / 0.5 h / 0 °C 12: 90 percent / sodium acetate, pyridinium chlorochromate / CH2Cl2 / 2 h 13: 33.8 percent / LDA, ZnCl2 / diethyl ether / 0 °C 14: H2 / palladium on carbon / ethanol / 3 h / Ambient temperature View Scheme |
-
-
505-10-2
3-(methylthio)-1-propanol

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| With ethanol; dicyclohexyl-carbodiimide In diethyl ether for 5h; Heating; | 84% |
-
-
75507-26-5
2-(hydroxymethyl)-12-crown-4

-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In diethyl ether for 5h; Heating; | 82% |
-
-
100-14-1
4-nitrobenzyl chloride

-
-
25999-31-9
lasalocid (X 537 A)

-
-
1026999-92-7
p-nitrobenzyl ester of lasalocid acid

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In toluene at 90℃; for 5h; | 75% |

| Conditions | Yield |
|---|---|
| With formaldehyd In toluene for 5h; Mannich Aminomethylation; Reflux; | 75% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In diethyl ether for 0.5h; Heating; | 74% |
-
-
25999-31-9
lasalocid (X 537 A)

-
-
112-27-6
2,2'-[1,2-ethanediylbis(oxy)]bisethanol

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In diethyl ether for 0.5h; Heating; | 71% |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In toluene at 90℃; for 5h; | 66% |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In toluene at 90℃; for 5h; | 60% |
-
-
612-23-7
2-nitrobenzyl chloride

-
-
25999-31-9
lasalocid (X 537 A)

-
-
1026999-90-5
o-nitrobenzyl ester of lasalocid acid

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In toluene at 90℃; for 5h; | 57% |
-
-
939-26-4
2-bromomethylnaphthyl bromide

-
-
25999-31-9
lasalocid (X 537 A)

-
-
1123683-88-4
lasalocid 2-naphthylmethyl ester

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In toluene at 90℃; for 5h; | 55% |
-
-
25999-31-9
lasalocid (X 537 A)

-
-
619-23-8
3-Nitrobenzyl chloride

-
-
1026999-91-6
m-nitrobenzyl ester of lasalocid acid

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In toluene at 90℃; for 5h; | 47% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In N,N-dimethyl-formamide at 20℃; for 24h; Inert atmosphere; | 42% |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In toluene at 90℃; for 5h; | 40% |
-
-
25999-31-9
lasalocid (X 537 A)

-
A
-
31478-26-9, 87678-18-0
4(R)-<5(S)-ethyl-3(S)-methyl-5-(5(R)-ethyl-5-hydroxy-6(S)-methyl-2(R)-tetrahydropyranyl)-2(S)-tetrahydrofuryl>hexan-3-one

-
B
-
73657-54-2
2-Hydroxy-3-methyl-6-((R)-3-methyl-4-oxo-butyl)-benzoic acid

| Conditions | Yield |
|---|---|
| Product distribution; heat or base treatment; |
-
-
67-56-1
methanol

-
-
25999-31-9
lasalocid (X 537 A)

-
-
33855-15-1
6-{(3R,4S,5S,7R)-7-[(2S,3S,5S)-5-Ethyl-5-((2R,5R,6S)-5-ethyl-5-hydroxy-6-methyl-tetrahydro-pyran-2-yl)-3-methyl-tetrahydro-furan-2-yl]-4-hydroxy-3,5-dimethyl-6-oxo-nonyl}-2-hydroxy-3-methyl-benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide at 25℃; for 48h; |
-
-
57260-73-8
N-BOC-1,2-diaminoethane

-
-
25999-31-9
lasalocid (X 537 A)

-
-
178912-24-8
[2-(6-{(3R,4S,5S,7R)-7-[(2S,3S,5S)-5-Ethyl-5-((2R,5R,6S)-5-ethyl-5-hydroxy-6-methyl-tetrahydro-pyran-2-yl)-3-methyl-tetrahydro-furan-2-yl]-4-hydroxy-3,5-dimethyl-6-oxo-nonyl}-2-hydroxy-3-methyl-benzoylamino)-ethyl]-carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With pyridine; benzotriazol-1-ol; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In N,N-dimethyl-formamide at 25℃; for 24h; |
-
-
25999-31-9
lasalocid (X 537 A)

-
-
178912-25-9
N-(2-Amino-ethyl)-6-{(3R,4S,5S,7R)-7-[(2S,3S,5S)-5-ethyl-5-((2R,5R,6S)-5-ethyl-5-hydroxy-6-methyl-tetrahydro-pyran-2-yl)-3-methyl-tetrahydro-furan-2-yl]-4-hydroxy-3,5-dimethyl-6-oxo-nonyl}-2-hydroxy-3-methyl-benzamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: EDC, HOBt, pyridine / dimethylformamide / 24 h / 25 °C 2: TFA / CH2Cl2 View Scheme |
-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: EDC, HOBt, pyridine / dimethylformamide / 24 h / 25 °C 2: TFA / CH2Cl2 3: EDC, HOBt, pyridine / dimethylformamide / 48 h / 25 °C View Scheme |
-
-
25999-31-9
lasalocid (X 537 A)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: EDC, HOBt, pyridine / dimethylformamide / 24 h / 25 °C 2: TFA / CH2Cl2 3: EDC, HOBt, pyridine / dimethylformamide / 48 h / 25 °C View Scheme |
Related products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View