Dayang Chem (Hangzhou) Co.,Ltd.
Dayangchem's R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. DayangChem can provide different quantities
Cas:72962-43-7
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquirySimagchem Corporation
Welcome to Simagchem, your partner in China as a premier supply of bulk specialty chemicals for industry and life science. We introduce experienced quality product and exceptional JIT service with instant market intelligence in China to benefit our
Cas:72962-43-7
Min.Order:0 Metric Ton
Negotiable
Type:Manufacturers
inquiryHefei TNJ chemical industry co.,ltd
Novel PGR for seed soaking or stem and leaves spraying. 1. Prompt growth 2. Improve quality 3. Increase quantity 4. Bright colors of leaves and flowers, thicken the foliar 5. Enhance weight and sugar content in fruit. 6. Advance time fo
Ality Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Xi'an Xszo Chem Co., Ltd.
1. Factory price and high quality must be guaranteed, base on 8 years of production and R&D experience2. Free samples will be provided,ensure specifications and quality are right for customer3. Customers will receive the most professional technical s
Cas:72962-43-7
Min.Order:1 Gram
FOB Price: $0.1
Type:Manufacturers
inquiryCOLORCOM LTD.
Colorcom is a global leader in industrial chemical manufacturing and is continuously innovating and transforming to exceed client expectations and industry standards. Colorcom prides itself on superior customer and technical focus, while focusing on
Chemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Cas:72962-43-7
Min.Order:5 Kiloliter
FOB Price: $1.2 / 5.0
Type:Manufacturers
inquiryHubei DiBo chemical co., LTD
Name: epibrassinolide CAS no. : 72962-43-7 Molecular formula: C28H48O6 Molecular weight:480.6771 Product Quality 12 years of chemical raw materials Mature operation of the industry System stability Data storage Security without vulnerabil
Cas:72962-43-7
Min.Order:25 Kilogram
FOB Price: $1.0 / 2.0
Type:Other
inquiryHebei Nengqian Chemical Import and Export Co., LTD
Our advantages: 1. All inquiries will be replied within 12 hours. 2. Dedication to quality, supply & service. 3. Strictly on selecting raw materials. 4. Reasonable & competitive price, fast lead time. 5. Sample is available for your eva
Cas:72962-43-7
Min.Order:1 Gram
FOB Price: $1000.0 / 1300.0
Type:Trading Company
inquiryHenan Tianfu Chemical Co., Ltd.
best service,quality & low price Our company was built in 2009 with an ISO certificate.In the past 5 years, we have grown up as a famous fine chemicals supplier in China and we had established stable business relationships with Samsung,LG,Mer
Cas:72962-43-7
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:72962-43-7
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryHenan Sinotech Import&Export Corporation
Product name Plant Growth Regulator Brassinolide(BR) 90% TC, 0.1%SP General info Function: Plant Growth Regulator
Shanghai Upbio Tech Co.,Ltd
1.No Less 8 years exporting experience. Clients can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specialized
Cas:72962-43-7
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryXi'an Faithful Biotech Co., Ltd.
We are the manufacturers and suppliers of API in China, and warehouse in Germany and USA of California, which can quickly and safely deliver to your address 1.High quality and competitive price. 2.Free sample for your evaluation. 3.Promptly delivery
Cas:72962-43-7
Min.Order:10 Gram
FOB Price: $1.32
Type:Trading Company
inquiryBaoji Guokang Healthchem co.,ltd
Our company has been in existence for 10 years since its establishment. We have our own unique team. The company integrates independent research and development, production and sales. We have established famous brands at home and abroad. At present
Cas:72962-43-7
Min.Order:10 Gram
FOB Price: $100.0 / 120.0
Type:Trading Company
inquiryQingdao Beluga Import and Export Co., LTD
Brassinolide CAS:72962-43-7 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organic intermediate
Cas:72962-43-7
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:72962-43-7
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHebei Mojin Biotechnology Co.,Ltd
Hebei Mojin Biotechnology Co., Ltd,Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all em
Cas:72962-43-7
Min.Order:1 Gram
FOB Price: $95.0 / 100.0
Type:Trading Company
inquiryChangchun Artel lmport and Export trade company
Product Detail Minimum Order Qty. 10 Gram
Cas:72962-43-7
Min.Order:20 Metric Ton
Negotiable
Type:Trading Company
inquiryLeader Biochemical Group
PRODUCT DETAILS
Cas:72962-43-7
Min.Order:500 Kilogram
FOB Price: $1.0 / 2.0
Type:Lab/Research institutions
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:72962-43-7
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryRely Chemicals Ltd.
1.quick response;2.competitive price with good quality product;3.flexible payment;4.fast delivery;5.superior after sell service. Appearance:White powder Package:as customerized Application:Promote the growth of plant to increase yield,etc . 2. K
Cas:72962-43-7
Min.Order:1 Kilogram
Negotiable
Type:Trading Company
inquirySHANGHAI T&W PHARMACEUTICAL CO., LTD.
A substitute for perfluorooctanoic acid, mainly used as a surfactant, dispersant, additive, etc Appearance:White solid or Colorless liquid Purity:99.3 % We will ship the goods in a timely manner as required We can provide relevant documents acc
Shanghai Massive Chemical Technology Co., Ltd.
Massive Chemical is certified with ISO9001 and ISO14001 manufacturer for this product. We will offer all documents as requirement for the materials which includes, Certificate of Analysis, Material Safety Data Sheet, and Method of Analysis and
Cas:72962-43-7
Min.Order:1 Gram
FOB Price: $1.0
Type:Lab/Research institutions
inquiryZibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:72962-43-7
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryHangzhou Lingrui Chemical Co.,Ltd.
High purity Brassinolide 72962-43-7 in stock immediately delivery good supplierAppearance:Powder Storage:Dry and ventilated Package:according to customers' requirements Application:APIs Transportation:By air(EMS or EUB or FedEx or TNT ect...) or by s
Cas:72962-43-7
Min.Order:1 Gram
Negotiable
Type:Other
inquirySiwei Development Group Ltd.
Product name: Epibrassinolide CAS No.:72962-43-7 Molecule Formula:C28H48O6 Molecule Weight:480.67 Purity: 98.0% Package: 25kg/drum Description:White powder Manufacture Standards:Enterprise Standard TESTING ITEMS S
Cas:72962-43-7
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryXiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
Hangzhou Fandachem Co.,Ltd
Hangzhou Fandachem Co.,Ltd, a China-based chemical company, specialize in exporting Brassinolide (BR)90%TC, 0.01%SP, 0.1%SP, CAS NO: 72962-43-7 Please contact us by email freely. We are leading exporter in China. If you really ne
Cas:72962-43-7
Min.Order:1 Kilogram
Negotiable
Type:Other
inquiryKAISA GROUP INC
1.Applied in food field.it can improve the immune system and prolong life. 2.Appliedin cosmetic field.it can improve the skin care. 3.Applied in pharmaceutical field.it can treat various dieases. 4.Our product quality assurance will make our customer
Cas:72962-43-7
Min.Order:1 Metric Ton
FOB Price: $7.0 / 8.0
Type:Trading Company
inquirySynthetic route
-
-
76987-58-1
2α,3α-22R,23R-tetraacetoxy-B-homo-7-oxa-24S-methyl-5α-cholestan-6-one

-
-
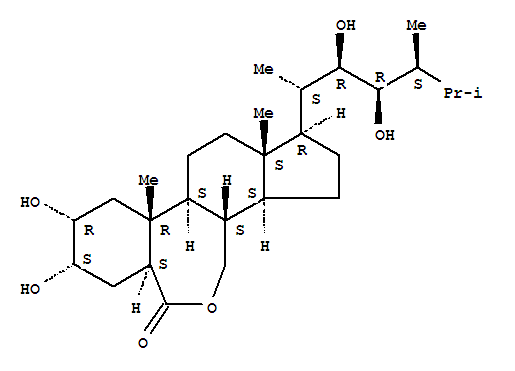
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol for 1h; Heating; | 94% |
| With sodium hydroxide In methanol; water for 3h; Heating; | 57% |
| With hydrogenchloride; methanol; potassium hydroxide 1.) reflux, 1 h, 2.) room temperature, 1 h; Yield given. Multistep reaction; | |
| Multi-step reaction with 2 steps 1: NaOH 2: HCl View Scheme |
-
-
83066-72-2
(2R,3S,5S,8S,9S,10R,13S,14S,17R)-17-{(S)-1-[(4R,5R)-5-((S)-1,2-Dimethyl-propyl)-2,2-dimethyl-[1,3]dioxolan-4-yl]-ethyl}-2,3-dihydroxy-10,13-dimethyl-hexadecahydro-cyclopenta[a]phenanthren-6-one

-
A
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

-
B
-
146205-07-4
(22R,23R,24S)-2α,3α,22,23-tetrahydroxy-6-oxa-7a-homo-5α-campestan-7-one

| Conditions | Yield |
|---|---|
| With trifluoroacetyl peroxide In dichloromethane at 22℃; for 1h; | A 74% B n/a |
-
-
91708-76-8
(2R,3S,5α,22R,23R,24S)-6,6-Ethylenedioxy-2,3-isopropylidenedioxyergostane-22,23-diol

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| With trifluoroacetyl peroxide; trifluoroacetic acid In chloroform for 3h; Ambient temperature; | 53% |
| Multi-step reaction with 3 steps 1: 7.62 g / 80percent AcOH / H2O / 1 h / 50 - 60 °C 2: pyridine, N,N-dimethylaminopyridine View Scheme |
-
-
124853-28-7
3-dehydroteasterone

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| With amalgamated zinc; osmium(VIII) oxide; chloro-trimethyl-silane; trifluoroacetyl peroxide; 4-methylmorpholine N-oxide 1.) THF, rt., 12 h; 2.) THF, aq. t-BuOH; 3.) CH2Cl2; Yield given. Multistep reaction; |
-
-
80736-41-0
castasterone

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction; | |
| Multi-step reaction with 2 steps 1: pyridine, N,N-dimethylaminopyridine View Scheme |
-
-
77027-48-6
2,3,22,23-tetra-O-acetylcastasterone

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction; |
-
-
106315-18-8
Acetic acid (1R,2R,3S)-2-acetoxy-1-[(S)-1-((1R,3aS,3bS,6aS,8S,9R,10aR,10bS,12aS)-8,9-dihydroxy-10a,12a-dimethyl-6-oxo-hexadecahydro-5-oxa-benzo[3,4]cyclohepta[1,2-e]inden-1-yl)-ethyl]-3,4-dimethyl-pentyl ester

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol for 3h; Heating; | 1.79 g |

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
111118-49-1
(1R,3aS,3bS,5aS,6aS,9aR,10aR,10bS,12aS)-1-{(S)-1-[(4R,5R)-5-((S)-1,2-Dimethyl-propyl)-2,2-dimethyl-[1,3]dioxolan-4-yl]-ethyl}-8,8,10a,12a-tetramethyl-hexadecahydro-7,9-dioxa-dicyclopenta[a,h]phenanthren-5-one

-
A
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

-
B
-
146205-07-4
(22R,23R,24S)-2α,3α,22,23-tetrahydroxy-6-oxa-7a-homo-5α-campestan-7-one

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction. Yields of byproduct given; |
-
-
81481-15-4
(20S)-6,6-ethylenedioxy-2α,3α-isopropylidenedioxy-5α-pregnane-20-carbaldehyde

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 1.) n-BuLi, 2.) 30percent H2O2 / 1.) THF, -78 deg C, 3 h, 2.) acetic acid, room temperature, 15 h 2: 85 percent / Ti(O-iPr)4, (+)-L-diethyl tartrate, cumene hydroperoxide, 4 Angstroem MS / CH2Cl2 / 144 h / -25 °C 3: 82 percent / CuCN / diethyl ether / 1.) -78 deg C, 2 h, 2.) 0 deg C, 3 h 4: 53 percent / CF3CO3H, CF3CO2H / CHCl3 / 3 h / Ambient temperature View Scheme | |
| Multi-step reaction with 8 steps 1: n-BuLi / tetrahydrofuran; hexane / -50 to -60 deg C; -40 deg C, 1 h; -68 to - 65 deg C, 1 h; 0 deg C 2: 74.6 percent / 1.) triphenylphosphine, benzoic acid, diethyl azodicarboxylate; 2.) K2CO3 / 1.) THF, 5-10 deg C, 30 min; 2.) THF/methanol, reflux, 3 h 3: 44.3 g / H2, 1M NaBH4, ethylenediamine, nickel acetate / aq. ethanol / 5 h 4: 93 percent / m-chloroperbenzoic acid, Na2HPO4 / CH2Cl2 / 20 h / 5 °C 5: n-BuLi / hexane; cyclohexane / -70 deg C, 1 h; 10 deg C, 4 h 6: 7.62 g / 80percent AcOH / H2O / 1 h / 50 - 60 °C 7: pyridine, N,N-dimethylaminopyridine View Scheme | |
| Multi-step reaction with 7 steps 1: 26.93 g / n-BuLi / tetrahydrofuran; hexane / -50 to -60 deg C; -40 deg C, 1 h; -68 to - 65 deg C, 1 h; 0 deg C 2: 44.3 g / H2, 1M NaBH4, ethylenediamine, nickel acetate / aq. ethanol / 5 h 3: 93 percent / m-chloroperbenzoic acid, Na2HPO4 / CH2Cl2 / 20 h / 5 °C 4: n-BuLi / hexane; cyclohexane / -70 deg C, 1 h; 10 deg C, 4 h 5: 7.62 g / 80percent AcOH / H2O / 1 h / 50 - 60 °C 6: pyridine, N,N-dimethylaminopyridine View Scheme |
-
-
148705-11-7
(2α,3α,5α,22S,23E)-6,6-ethylenedioxy-2,3-isopropylidenedioxy-26,27-dinorcholest-23-en-22-ol

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 85 percent / Ti(O-iPr)4, (+)-L-diethyl tartrate, cumene hydroperoxide, 4 Angstroem MS / CH2Cl2 / 144 h / -25 °C 2: 82 percent / CuCN / diethyl ether / 1.) -78 deg C, 2 h, 2.) 0 deg C, 3 h 3: 53 percent / CF3CO3H, CF3CO2H / CHCl3 / 3 h / Ambient temperature View Scheme |
-
-
223117-11-1
(2R,3S,5α,22R)-23,24-epoxy-6,6-(ethylenedioxy)-2,3-(isopropylidenedioxy)-26,27-dinorcholestan-22-ol

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 82 percent / CuCN / diethyl ether / 1.) -78 deg C, 2 h, 2.) 0 deg C, 3 h 2: 53 percent / CF3CO3H, CF3CO2H / CHCl3 / 3 h / Ambient temperature View Scheme |
-
-
563-80-4
3-methyl-butan-2-one

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: Br2 / methanol 2: acetone / 18 h / Heating 3: NaH / tetrahydrofuran / 0.5 h / 25 °C 4: 80 percent / LDA / tetrahydrofuran / 1.) -40 to -50 deg C, 30 min, 2.) -75 deg C, 14 h 5: 83 percent / H2, pyridine / 10percent Pd/C / ethanol; tetrahydrofuran / 14 h / 2585.7 Torr / Ambient temperature 6: 99 percent / LAH / tetrahydrofuran / 1.) r.t. 2 h, 2.) reflux, overnight 7: 96 percent / CSA / 24 h / Ambient temperature 8: 91 percent / CrO3, pyridine / CH2Cl2 / 48 h / Ambient temperature 9: 72 percent / <(C6H5)3P>3RhCl / benzene / 5 h / Heating View Scheme | |
| Multi-step reaction with 10 steps 1: Br2 / methanol 2: acetone / 18 h / Heating 3: 1.) NaH, triethylphosphonoacetate, 2.) aq. KOH / 1.) THF, 25 deg C, 30 min, 2.) 25 deg C, 24 h 4: 80 percent / LDA / tetrahydrofuran / 1.) -40 to -50 deg C, 30 min, 2.) -75 deg C, 14 h 5: 83 percent / H2, pyridine / 10percent Pd/C / ethanol; tetrahydrofuran / 14 h / 2585.7 Torr / Ambient temperature 6: 99 percent / LAH / tetrahydrofuran / 1.) r.t. 2 h, 2.) reflux, overnight 7: 96 percent / CSA / 24 h / Ambient temperature 8: 91 percent / CrO3, pyridine / CH2Cl2 / 48 h / Ambient temperature 9: 72 percent / <(C6H5)3P>3RhCl / benzene / 5 h / Heating View Scheme |
-
-
10547-89-4
4-(1-methylethyl)furan-2(5H)-one

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 80 percent / LDA / tetrahydrofuran / 1.) -40 to -50 deg C, 30 min, 2.) -75 deg C, 14 h 2: 83 percent / H2, pyridine / 10percent Pd/C / ethanol; tetrahydrofuran / 14 h / 2585.7 Torr / Ambient temperature 3: 99 percent / LAH / tetrahydrofuran / 1.) r.t. 2 h, 2.) reflux, overnight 4: 96 percent / CSA / 24 h / Ambient temperature 5: 91 percent / CrO3, pyridine / CH2Cl2 / 48 h / Ambient temperature 6: 72 percent / <(C6H5)3P>3RhCl / benzene / 5 h / Heating View Scheme |
-
-
36960-07-3
1-(acetyloxy)-3-methyl-2-butanone

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: NaH / tetrahydrofuran / 0.5 h / 25 °C 2: 80 percent / LDA / tetrahydrofuran / 1.) -40 to -50 deg C, 30 min, 2.) -75 deg C, 14 h 3: 83 percent / H2, pyridine / 10percent Pd/C / ethanol; tetrahydrofuran / 14 h / 2585.7 Torr / Ambient temperature 4: 99 percent / LAH / tetrahydrofuran / 1.) r.t. 2 h, 2.) reflux, overnight 5: 96 percent / CSA / 24 h / Ambient temperature 6: 91 percent / CrO3, pyridine / CH2Cl2 / 48 h / Ambient temperature 7: 72 percent / <(C6H5)3P>3RhCl / benzene / 5 h / Heating View Scheme | |
| Multi-step reaction with 8 steps 1: 1.) NaH, triethylphosphonoacetate, 2.) aq. KOH / 1.) THF, 25 deg C, 30 min, 2.) 25 deg C, 24 h 2: 80 percent / LDA / tetrahydrofuran / 1.) -40 to -50 deg C, 30 min, 2.) -75 deg C, 14 h 3: 83 percent / H2, pyridine / 10percent Pd/C / ethanol; tetrahydrofuran / 14 h / 2585.7 Torr / Ambient temperature 4: 99 percent / LAH / tetrahydrofuran / 1.) r.t. 2 h, 2.) reflux, overnight 5: 96 percent / CSA / 24 h / Ambient temperature 6: 91 percent / CrO3, pyridine / CH2Cl2 / 48 h / Ambient temperature 7: 72 percent / <(C6H5)3P>3RhCl / benzene / 5 h / Heating View Scheme |
-
-
74174-45-1
(22E)-stigmasta-2,22-dien-6-one

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1: 72 percent / OsO4, NMO, H2O / acetone; 2-methyl-propan-2-ol / 12 h / Ambient temperature 2: 98 percent / CSA / acetonitrile; CH2Cl2 / 1 h / Ambient temperature 3: OsO4, NMO, aq. NaHCO3, MeSO2NH2 / tetrahydrofuran; 2-methyl-propan-2-ol / 144 h / 40 °C 4: 82 percent / pyridine, NaHCO3, H5IO6 / tetrahydrofuran / 24 h / Ambient temperature 5: 80 percent / LDA / tetrahydrofuran / 1.) -40 to -50 deg C, 30 min, 2.) -75 deg C, 14 h 6: 83 percent / H2, pyridine / 10percent Pd/C / ethanol; tetrahydrofuran / 14 h / 2585.7 Torr / Ambient temperature 7: 99 percent / LAH / tetrahydrofuran / 1.) r.t. 2 h, 2.) reflux, overnight 8: 96 percent / CSA / 24 h / Ambient temperature 9: 91 percent / CrO3, pyridine / CH2Cl2 / 48 h / Ambient temperature 10: 72 percent / <(C6H5)3P>3RhCl / benzene / 5 h / Heating View Scheme |
-
-
74174-49-5
(22E,24S)-3β,5-cyclo-24-ethylcholest-22-en-6-one

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1: 80 percent / pyridine hydrochloride, LiBr / N,N-dimethyl-acetamide / 4 h / 160 °C 2: 72 percent / OsO4, NMO, H2O / acetone; 2-methyl-propan-2-ol / 12 h / Ambient temperature 3: 98 percent / CSA / acetonitrile; CH2Cl2 / 1 h / Ambient temperature 4: OsO4, NMO, aq. NaHCO3, MeSO2NH2 / tetrahydrofuran; 2-methyl-propan-2-ol / 144 h / 40 °C 5: 82 percent / pyridine, NaHCO3, H5IO6 / tetrahydrofuran / 24 h / Ambient temperature 6: 80 percent / LDA / tetrahydrofuran / 1.) -40 to -50 deg C, 30 min, 2.) -75 deg C, 14 h 7: 83 percent / H2, pyridine / 10percent Pd/C / ethanol; tetrahydrofuran / 14 h / 2585.7 Torr / Ambient temperature 8: 99 percent / LAH / tetrahydrofuran / 1.) r.t. 2 h, 2.) reflux, overnight 9: 96 percent / CSA / 24 h / Ambient temperature 10: 91 percent / CrO3, pyridine / CH2Cl2 / 48 h / Ambient temperature 11: 72 percent / <(C6H5)3P>3RhCl / benzene / 5 h / Heating View Scheme |
-
-
81481-12-1
(22E,24S)-2α,3α-dihydroxy-5α-stigmast-22-en-6-one

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 98 percent / CSA / acetonitrile; CH2Cl2 / 1 h / Ambient temperature 2: OsO4, NMO, aq. NaHCO3, MeSO2NH2 / tetrahydrofuran; 2-methyl-propan-2-ol / 144 h / 40 °C 3: 82 percent / pyridine, NaHCO3, H5IO6 / tetrahydrofuran / 24 h / Ambient temperature 4: 80 percent / LDA / tetrahydrofuran / 1.) -40 to -50 deg C, 30 min, 2.) -75 deg C, 14 h 5: 83 percent / H2, pyridine / 10percent Pd/C / ethanol; tetrahydrofuran / 14 h / 2585.7 Torr / Ambient temperature 6: 99 percent / LAH / tetrahydrofuran / 1.) r.t. 2 h, 2.) reflux, overnight 7: 96 percent / CSA / 24 h / Ambient temperature 8: 91 percent / CrO3, pyridine / CH2Cl2 / 48 h / Ambient temperature 9: 72 percent / <(C6H5)3P>3RhCl / benzene / 5 h / Heating View Scheme |
-
-
81481-13-2
(22E,24S)-2α,3α-Dihydroxy-5α-stigmast-22-en-6-one 2,3-acetonide

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: OsO4, NMO, aq. NaHCO3, MeSO2NH2 / tetrahydrofuran; 2-methyl-propan-2-ol / 144 h / 40 °C 2: 82 percent / pyridine, NaHCO3, H5IO6 / tetrahydrofuran / 24 h / Ambient temperature 3: 80 percent / LDA / tetrahydrofuran / 1.) -40 to -50 deg C, 30 min, 2.) -75 deg C, 14 h 4: 83 percent / H2, pyridine / 10percent Pd/C / ethanol; tetrahydrofuran / 14 h / 2585.7 Torr / Ambient temperature 5: 99 percent / LAH / tetrahydrofuran / 1.) r.t. 2 h, 2.) reflux, overnight 6: 96 percent / CSA / 24 h / Ambient temperature 7: 91 percent / CrO3, pyridine / CH2Cl2 / 48 h / Ambient temperature 8: 72 percent / <(C6H5)3P>3RhCl / benzene / 5 h / Heating View Scheme | |
| Multi-step reaction with 10 steps 1: 83.0 g / p-TsOH / 3 h / Heating 2: 30 g / ozone, NaHCO3 / methanol; CH2Cl2 / 2 h / -60 - -50 °C 3: n-BuLi / tetrahydrofuran; hexane / -50 to -60 deg C; -40 deg C, 1 h; -68 to - 65 deg C, 1 h; 0 deg C 4: 74.6 percent / 1.) triphenylphosphine, benzoic acid, diethyl azodicarboxylate; 2.) K2CO3 / 1.) THF, 5-10 deg C, 30 min; 2.) THF/methanol, reflux, 3 h 5: 44.3 g / H2, 1M NaBH4, ethylenediamine, nickel acetate / aq. ethanol / 5 h 6: 93 percent / m-chloroperbenzoic acid, Na2HPO4 / CH2Cl2 / 20 h / 5 °C 7: n-BuLi / hexane; cyclohexane / -70 deg C, 1 h; 10 deg C, 4 h 8: 7.62 g / 80percent AcOH / H2O / 1 h / 50 - 60 °C 9: pyridine, N,N-dimethylaminopyridine View Scheme | |
| Multi-step reaction with 9 steps 1: 83.0 g / p-TsOH / 3 h / Heating 2: 30 g / ozone, NaHCO3 / methanol; CH2Cl2 / 2 h / -60 - -50 °C 3: 26.93 g / n-BuLi / tetrahydrofuran; hexane / -50 to -60 deg C; -40 deg C, 1 h; -68 to - 65 deg C, 1 h; 0 deg C 4: 44.3 g / H2, 1M NaBH4, ethylenediamine, nickel acetate / aq. ethanol / 5 h 5: 93 percent / m-chloroperbenzoic acid, Na2HPO4 / CH2Cl2 / 20 h / 5 °C 6: n-BuLi / hexane; cyclohexane / -70 deg C, 1 h; 10 deg C, 4 h 7: 7.62 g / 80percent AcOH / H2O / 1 h / 50 - 60 °C 8: pyridine, N,N-dimethylaminopyridine View Scheme |
-
-
103881-47-6
(22E,24S)-24-ethyl-3α,5-cyclo-5α-cholest-22-en-6-ol

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1: CrO3, H2SO4 / acetone 2: 80 percent / pyridine hydrochloride, LiBr / N,N-dimethyl-acetamide / 4 h / 160 °C 3: 72 percent / OsO4, NMO, H2O / acetone; 2-methyl-propan-2-ol / 12 h / Ambient temperature 4: 98 percent / CSA / acetonitrile; CH2Cl2 / 1 h / Ambient temperature 5: OsO4, NMO, aq. NaHCO3, MeSO2NH2 / tetrahydrofuran; 2-methyl-propan-2-ol / 144 h / 40 °C 6: 82 percent / pyridine, NaHCO3, H5IO6 / tetrahydrofuran / 24 h / Ambient temperature 7: 80 percent / LDA / tetrahydrofuran / 1.) -40 to -50 deg C, 30 min, 2.) -75 deg C, 14 h 8: 83 percent / H2, pyridine / 10percent Pd/C / ethanol; tetrahydrofuran / 14 h / 2585.7 Torr / Ambient temperature 9: 99 percent / LAH / tetrahydrofuran / 1.) r.t. 2 h, 2.) reflux, overnight 10: 96 percent / CSA / 24 h / Ambient temperature 11: 91 percent / CrO3, pyridine / CH2Cl2 / 48 h / Ambient temperature 12: 72 percent / <(C6H5)3P>3RhCl / benzene / 5 h / Heating View Scheme |
-
-
19967-55-6
1-bromo-3-methyl-2-butanone

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: acetone / 18 h / Heating 2: NaH / tetrahydrofuran / 0.5 h / 25 °C 3: 80 percent / LDA / tetrahydrofuran / 1.) -40 to -50 deg C, 30 min, 2.) -75 deg C, 14 h 4: 83 percent / H2, pyridine / 10percent Pd/C / ethanol; tetrahydrofuran / 14 h / 2585.7 Torr / Ambient temperature 5: 99 percent / LAH / tetrahydrofuran / 1.) r.t. 2 h, 2.) reflux, overnight 6: 96 percent / CSA / 24 h / Ambient temperature 7: 91 percent / CrO3, pyridine / CH2Cl2 / 48 h / Ambient temperature 8: 72 percent / <(C6H5)3P>3RhCl / benzene / 5 h / Heating View Scheme | |
| Multi-step reaction with 9 steps 1: acetone / 18 h / Heating 2: 1.) NaH, triethylphosphonoacetate, 2.) aq. KOH / 1.) THF, 25 deg C, 30 min, 2.) 25 deg C, 24 h 3: 80 percent / LDA / tetrahydrofuran / 1.) -40 to -50 deg C, 30 min, 2.) -75 deg C, 14 h 4: 83 percent / H2, pyridine / 10percent Pd/C / ethanol; tetrahydrofuran / 14 h / 2585.7 Torr / Ambient temperature 5: 99 percent / LAH / tetrahydrofuran / 1.) r.t. 2 h, 2.) reflux, overnight 6: 96 percent / CSA / 24 h / Ambient temperature 7: 91 percent / CrO3, pyridine / CH2Cl2 / 48 h / Ambient temperature 8: 72 percent / <(C6H5)3P>3RhCl / benzene / 5 h / Heating View Scheme |
-
-
174656-33-8
(20S)-2α,3α-isopropylidenedioxy-5α-pregnan-6-one-20-carbaldehyde

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 80 percent / LDA / tetrahydrofuran / 1.) -40 to -50 deg C, 30 min, 2.) -75 deg C, 14 h 2: 83 percent / H2, pyridine / 10percent Pd/C / ethanol; tetrahydrofuran / 14 h / 2585.7 Torr / Ambient temperature 3: 99 percent / LAH / tetrahydrofuran / 1.) r.t. 2 h, 2.) reflux, overnight 4: 96 percent / CSA / 24 h / Ambient temperature 5: 91 percent / CrO3, pyridine / CH2Cl2 / 48 h / Ambient temperature 6: 72 percent / <(C6H5)3P>3RhCl / benzene / 5 h / Heating View Scheme |

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 96 percent / CSA / 24 h / Ambient temperature 2: 91 percent / CrO3, pyridine / CH2Cl2 / 48 h / Ambient temperature 3: 72 percent / <(C6H5)3P>3RhCl / benzene / 5 h / Heating View Scheme |

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 91 percent / CrO3, pyridine / CH2Cl2 / 48 h / Ambient temperature 2: 72 percent / <(C6H5)3P>3RhCl / benzene / 5 h / Heating View Scheme |
-
-
174656-38-3
(S)-3-{(4R,5R)-2,2-Dimethyl-5-[(S)-1-((1R,3aS,3bS,5aS,6aS,9aR,10aR,10bS,12aS)-8,8,10a,12a-tetramethyl-5-oxo-hexadecahydro-7,9-dioxa-dicyclopenta[a,h]phenanthren-1-yl)-ethyl]-[1,3]dioxolan-4-yl}-4-methyl-pentanal

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 72 percent / <(C6H5)3P>3RhCl / benzene / 5 h / Heating View Scheme |
-
-
123450-38-4
(1R,3aS,3bS,5aS,6aS,9aR,10aR,10bS,12aS)-1-((1S,2S,3S,4S)-4-Ethyl-2,3-dihydroxy-1,5-dimethyl-hexyl)-8,8,10a,12a-tetramethyl-hexadecahydro-7,9-dioxa-dicyclopenta[a,h]phenanthren-5-one

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 82 percent / pyridine, NaHCO3, H5IO6 / tetrahydrofuran / 24 h / Ambient temperature 2: 80 percent / LDA / tetrahydrofuran / 1.) -40 to -50 deg C, 30 min, 2.) -75 deg C, 14 h 3: 83 percent / H2, pyridine / 10percent Pd/C / ethanol; tetrahydrofuran / 14 h / 2585.7 Torr / Ambient temperature 4: 99 percent / LAH / tetrahydrofuran / 1.) r.t. 2 h, 2.) reflux, overnight 5: 96 percent / CSA / 24 h / Ambient temperature 6: 91 percent / CrO3, pyridine / CH2Cl2 / 48 h / Ambient temperature 7: 72 percent / <(C6H5)3P>3RhCl / benzene / 5 h / Heating View Scheme |
-
-
174656-35-0
(1R,3aS,3bS,5aS,6aS,9aR,10aR,10bS,12aS)-1-[(1S,2R)-2-Hydroxy-2-((2R,3S)-3-isopropyl-5-oxo-tetrahydro-furan-2-yl)-1-methyl-ethyl]-8,8,10a,12a-tetramethyl-hexadecahydro-7,9-dioxa-dicyclopenta[a,h]phenanthren-5-one

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 99 percent / LAH / tetrahydrofuran / 1.) r.t. 2 h, 2.) reflux, overnight 2: 96 percent / CSA / 24 h / Ambient temperature 3: 91 percent / CrO3, pyridine / CH2Cl2 / 48 h / Ambient temperature 4: 72 percent / <(C6H5)3P>3RhCl / benzene / 5 h / Heating View Scheme |
-
-
174656-34-9
(1R,3aS,3bS,5aS,6aS,9aR,10aR,10bS,12aS)-1-[(1S,2R)-2-Hydroxy-2-((R)-3-isopropyl-5-oxo-2,5-dihydro-furan-2-yl)-1-methyl-ethyl]-8,8,10a,12a-tetramethyl-hexadecahydro-7,9-dioxa-dicyclopenta[a,h]phenanthren-5-one

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 83 percent / H2, pyridine / 10percent Pd/C / ethanol; tetrahydrofuran / 14 h / 2585.7 Torr / Ambient temperature 2: 99 percent / LAH / tetrahydrofuran / 1.) r.t. 2 h, 2.) reflux, overnight 3: 96 percent / CSA / 24 h / Ambient temperature 4: 91 percent / CrO3, pyridine / CH2Cl2 / 48 h / Ambient temperature 5: 72 percent / <(C6H5)3P>3RhCl / benzene / 5 h / Heating View Scheme |
-
-
81481-14-3
6,6-ethylenedioxy-2α,3α-isopropylidenedioxy-24S-ethyl-5α-cholest-22E-ene

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 30 g / ozone, NaHCO3 / methanol; CH2Cl2 / 2 h / -60 - -50 °C 2: n-BuLi / tetrahydrofuran; hexane / -50 to -60 deg C; -40 deg C, 1 h; -68 to - 65 deg C, 1 h; 0 deg C 3: 74.6 percent / 1.) triphenylphosphine, benzoic acid, diethyl azodicarboxylate; 2.) K2CO3 / 1.) THF, 5-10 deg C, 30 min; 2.) THF/methanol, reflux, 3 h 4: 44.3 g / H2, 1M NaBH4, ethylenediamine, nickel acetate / aq. ethanol / 5 h 5: 93 percent / m-chloroperbenzoic acid, Na2HPO4 / CH2Cl2 / 20 h / 5 °C 6: n-BuLi / hexane; cyclohexane / -70 deg C, 1 h; 10 deg C, 4 h 7: 7.62 g / 80percent AcOH / H2O / 1 h / 50 - 60 °C 8: pyridine, N,N-dimethylaminopyridine View Scheme | |
| Multi-step reaction with 8 steps 1: 30 g / ozone, NaHCO3 / methanol; CH2Cl2 / 2 h / -60 - -50 °C 2: 26.93 g / n-BuLi / tetrahydrofuran; hexane / -50 to -60 deg C; -40 deg C, 1 h; -68 to - 65 deg C, 1 h; 0 deg C 3: 44.3 g / H2, 1M NaBH4, ethylenediamine, nickel acetate / aq. ethanol / 5 h 4: 93 percent / m-chloroperbenzoic acid, Na2HPO4 / CH2Cl2 / 20 h / 5 °C 5: n-BuLi / hexane; cyclohexane / -70 deg C, 1 h; 10 deg C, 4 h 6: 7.62 g / 80percent AcOH / H2O / 1 h / 50 - 60 °C 7: pyridine, N,N-dimethylaminopyridine View Scheme |
-
-
86413-56-1
(2R, 3S, 22R, 23S, 24R)-23,24-epoxy-6,6-ethylenedioxy-22-hydroxy-2,3-isopropylidenedioxy-5α-cholestane

-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: n-BuLi / hexane; cyclohexane / -70 deg C, 1 h; 10 deg C, 4 h 2: 7.62 g / 80percent AcOH / H2O / 1 h / 50 - 60 °C 3: pyridine, N,N-dimethylaminopyridine View Scheme |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

-
-
108-24-7
acetic anhydride

-
-
76987-58-1
2α,3α-22R,23R-tetraacetoxy-B-homo-7-oxa-24S-methyl-5α-cholestan-6-one

| Conditions | Yield |
|---|---|
| With pyridine at 60℃; for 20h; Acetylation; | 95% |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

-
-
67-64-1
acetone

-
-
220399-01-9
C34H56O6

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid for 8h; Ambient temperature; | 91% |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

-
-
77-76-9
2,2-dimethoxy-propane

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In dichloromethane Ambient temperature; | 82% |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| With methanol; Dowex 50W resin (H(1+)-form); water; sodium methylate 1.) reflux, 5 h, 2.) MeOH, r.t., 3 h; Yield given. Multistep reaction. Yields of byproduct given; |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

-
-
180961-17-5
2,3,22,33-tetra-O-acetyl-25-hydroxybrassinolide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 95 percent / pyridine / 20 h / 60 °C 2: 61 percent / methyl(trifluoromethyl)dioxirane; 1,1,1-trifluoroacetone / CH2Cl2 / 5 h / -30 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 95 percent / pyridine / 20 h / 60 °C 2: 48 percent / methyl(trifluoromethyl)dioxirane; 1,1,1-trifluoroacetone / CH2Cl2 / 3 h / 0 °C View Scheme |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 95 percent / pyridine / 20 h / 60 °C 2: 61 percent / methyl(trifluoromethyl)dioxirane; 1,1,1-trifluoroacetone / CH2Cl2 / 5 h / -30 °C 3: 86 percent / KOH / methanol; H2O / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 95 percent / pyridine / 20 h / 60 °C 2: 61 percent / methyl(trifluoromethyl)dioxirane; 1,1,1-trifluoroacetone / CH2Cl2 / 5 h / -30 °C 3: 56 percent / KOH / methanol; H2O / 3 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: 95 percent / pyridine / 20 h / 60 °C 2: 48 percent / methyl(trifluoromethyl)dioxirane; 1,1,1-trifluoroacetone / CH2Cl2 / 3 h / 0 °C 3: 86 percent / KOH / methanol; H2O / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 95 percent / pyridine / 20 h / 60 °C 2: 48 percent / methyl(trifluoromethyl)dioxirane; 1,1,1-trifluoroacetone / CH2Cl2 / 3 h / 0 °C 3: 56 percent / KOH / methanol; H2O / 3 h / Heating View Scheme |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 95 percent / pyridine / 20 h / 60 °C 2: 6.5 percent / methyl(trifluoromethyl)dioxirane; 1,1,1-trifluoroacetone / CH2Cl2 / 3 h / 0 °C 3: 82 percent / KOH / methanol; H2O / 1 h / 20 °C View Scheme |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 95 percent / pyridine / 20 h / 60 °C 2: 61 percent / methyl(trifluoromethyl)dioxirane; 1,1,1-trifluoroacetone / CH2Cl2 / 5 h / -30 °C 3: 12 percent / KOH / methanol; H2O / 3 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: 95 percent / pyridine / 20 h / 60 °C 2: 48 percent / methyl(trifluoromethyl)dioxirane; 1,1,1-trifluoroacetone / CH2Cl2 / 3 h / 0 °C 3: 12 percent / KOH / methanol; H2O / 3 h / Heating View Scheme |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 95 percent / pyridine / 20 h / 60 °C 2: 61 percent / methyl(trifluoromethyl)dioxirane; 1,1,1-trifluoroacetone / CH2Cl2 / 5 h / -30 °C 3: 22 percent / KOH / methanol; H2O / 3 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: 95 percent / pyridine / 20 h / 60 °C 2: 48 percent / methyl(trifluoromethyl)dioxirane; 1,1,1-trifluoroacetone / CH2Cl2 / 3 h / 0 °C 3: 22 percent / KOH / methanol; H2O / 3 h / Heating View Scheme |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 95 percent / pyridine / 20 h / 60 °C 2: 18 percent / methyl(trifluoromethyl)dioxirane; 1,1,1-trifluoroacetone / CH2Cl2 / 3 h / 0 °C 3: 79 percent / KOH / methanol; H2O / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 95 percent / pyridine / 20 h / 60 °C 2: 78 percent / methyl(trifluoromethyl)dioxirane; 1,1,1-trifluoroacetone / CH2Cl2 / 24 h / 20 °C 3: 79 percent / KOH / methanol; H2O / 1 h / 20 °C View Scheme |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 95 percent / pyridine / 20 h / 60 °C 2: 6.5 percent / methyl(trifluoromethyl)dioxirane; 1,1,1-trifluoroacetone / CH2Cl2 / 3 h / 0 °C View Scheme |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 95 percent / pyridine / 20 h / 60 °C 2: 3.6 percent / methyl(trifluoromethyl)dioxirane; 1,1,1-trifluoroacetone / CH2Cl2 / 3 h / 0 °C View Scheme |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

-
-
215502-60-6
2,3,22,33-tetra-O-acetyl-14α,25-dihydroxybrassinolide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 95 percent / pyridine / 20 h / 60 °C 2: 18 percent / methyl(trifluoromethyl)dioxirane; 1,1,1-trifluoroacetone / CH2Cl2 / 3 h / 0 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 95 percent / pyridine / 20 h / 60 °C 2: 78 percent / methyl(trifluoromethyl)dioxirane; 1,1,1-trifluoroacetone / CH2Cl2 / 24 h / 20 °C View Scheme |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

-
-
220398-86-7
(22R,23R,24S)-22,23-isopropylidenedioxy-B-homo-7-oxa-5α-ergost-2-en-6-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 91 percent / p-TsOH / 8 h / Ambient temperature 2: 86 percent / aq. p-TsOH / dioxane / 4 h / 60 °C 3: 4-dimethylaminopyridine / CH2Cl2 / 2 h / 0 °C 4: triethyl phosphite / 5 h / Heating View Scheme |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

-
-
220399-03-1
(22R,23R,24S)-2α,3α-epoxy-22,23-isopropylidenedioxy-B-homo-7-oxa-5α-ergostan-6-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 91 percent / p-TsOH / 8 h / Ambient temperature 2: 86 percent / aq. p-TsOH / dioxane / 4 h / 60 °C 3: 4-dimethylaminopyridine / CH2Cl2 / 2 h / 0 °C 4: triethyl phosphite / 5 h / Heating 5: 50 percent / m-CPBA / CH2Cl2 / 2 h / 0 °C View Scheme |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

-
-
220398-85-6
(22R,23R,24S)-2α,3α-dihydroxy-22,23-isopropylidenedioxy-B-homo-7-oxa-5α-ergostan-6-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 91 percent / p-TsOH / 8 h / Ambient temperature 2: 86 percent / aq. p-TsOH / dioxane / 4 h / 60 °C View Scheme |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

-
-
220398-88-9
(22R,23R,24S)-2α,3β-dihydroxy-22,23-isopropylidenedioxy-B-homo-7-oxa-5α-ergostan-6-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 91 percent / p-TsOH / 8 h / Ambient temperature 2: 86 percent / aq. p-TsOH / dioxane / 4 h / 60 °C 3: Et3N, DMAP / CH2Cl2 / 2 h / Ambient temperature 4: pyridinium dichromate, pyridine / 5 h / 70 °C 5: NaBH4 / tetrahydrofuran / 5 h / Ambient temperature 6: tetra-n-butylammonium fluoride / tetrahydrofuran / 1 h / Ambient temperature View Scheme |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

-
-
220401-52-5
(22R,23R,24S)-2β,3α,22,23-tetrahydroxy-B-homo-7-oxa-5α-ergostan-6-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 91 percent / p-TsOH / 8 h / Ambient temperature 2: 86 percent / aq. p-TsOH / dioxane / 4 h / 60 °C 3: 4-dimethylaminopyridine / CH2Cl2 / 2 h / 0 °C 4: triethyl phosphite / 5 h / Heating 5: 50 percent / m-CPBA / CH2Cl2 / 2 h / 0 °C 6: 30 percent / aq. HClO4 / dioxane / 5 h / Ambient temperature View Scheme |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 91 percent / p-TsOH / 8 h / Ambient temperature 2: 86 percent / aq. p-TsOH / dioxane / 4 h / 60 °C 3: Et3N, DMAP / CH2Cl2 / 2 h / Ambient temperature 4: pyridinium dichromate, pyridine / 5 h / 70 °C 5: NaBH4 / tetrahydrofuran / 5 h / Ambient temperature 6: tetra-n-butylammonium fluoride / tetrahydrofuran / 1 h / Ambient temperature 7: 85 percent / 80 percent aq. AcOH / 2 h / 100 °C View Scheme |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 91 percent / p-TsOH / 8 h / Ambient temperature 2: 86 percent / aq. p-TsOH / dioxane / 4 h / 60 °C 3: 4-dimethylaminopyridine / CH2Cl2 / 2 h / 0 °C View Scheme |
-
-
72962-43-7
(3α,5α,6α,8β,9α,12β,13β,16α,17β)-10-[(2'α,3'β,4'β,5'β)-3,4-dihydroxy-5,6-dimethyl-2-heptanyl]-5,6-dihydroxy-7a,9a-dimethylhexadecahydro-3H-benzo[c]indeno[5,4-e]oxepin-3-one

-
-
220398-87-8
(22R,23R,24S)-2α-t-butyldimethylsiloxy-22,23-isopropylidenedioxy-B-homo-7-oxa-5α-ergostan-3,6-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 91 percent / p-TsOH / 8 h / Ambient temperature 2: 86 percent / aq. p-TsOH / dioxane / 4 h / 60 °C 3: Et3N, DMAP / CH2Cl2 / 2 h / Ambient temperature 4: pyridinium dichromate, pyridine / 5 h / 70 °C View Scheme |
Related products
Raw Materials
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View