Hangzhou Think Chemical Co. Ltd
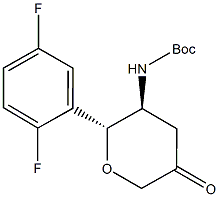
N-[(2R,3S)-2-(2,5-Difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester CAS No.:951127-25-6 Name: N-[(2R,3S)-2-(2,5-Difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-di
Cas:951127-25-6
Min.Order:1 Kilogram
FOB Price: $2.0
Type:Other
inquiryEnke Pharma-tech Co.,Ltd. (Cangzhou, China )
Cangzhou Enke Pharma Tech Co.,ltd. is located in Cangzhou City, Hebei province ,where is a famous petroleum chemical industry city in China. Enke Pharma a high-tech enterprise ,and we are dedicated to developing and manufacturing new ap
Dayang Chem (Hangzhou) Co.,Ltd.
Dayangchem’s R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. Near 20 years DayangChem has provided dif
Cas:951127-25-6
Min.Order:1 Gram
FOB Price: $3.0 / 4.0
Type:Lab/Research institutions
inquiryChemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Cas:951127-25-6
Min.Order:5 Kiloliter
FOB Price: $1.2 / 5.0
Type:Manufacturers
inquiryHenan Tianfu Chemical Co., Ltd.
Competitive Price High Quality Fast Delivery custom-made Welcome to Henan Tianfu Chemical Co., Ltd. website. Our company engages in Sodium Tripolyphosphate (STPP) and Sodium Hexametabphosphate (SHMP) production; development of noble me
Hebei Nengqian Chemical Import and Export Co., LTD
Our advantages: 1. All inquiries will be replied within 12 hours. 2. Dedication to quality, supply & service. 3. Strictly on selecting raw materials. 4. Reasonable & competitive price, fast lead time. 5. Sample is available for your eva
Cas:951127-25-6
Min.Order:1 Gram
FOB Price: $1000.0 / 1300.0
Type:Trading Company
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:951127-25-6
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryLIDE PHARMACEUTICALS LIMITED
Advantage : LIDE PHARMACEUTICALS LTD. is a mid-small manufacturing-type enterprise, engaged in pharmaceutical intermediates of R&D, custom-made and production, and also involving trading chemicals for export. We have established the
Cas:951127-25-6
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryQingdao Beluga Import and Export Co., LTD
CPo3604-01 CAS:951127-25-6 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organic intermediates
Cas:951127-25-6
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Henan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Triumph International Development Limilted
Appearance:white or light yellow crystalline powder Storage:Store in a cool,dry place and keep away from direct strong light Package:As customer request Application:Used for research and industrial manufacture. Transportation:By
Cas:951127-25-6
Min.Order:100 Gram
Negotiable
Type:Lab/Research institutions
inquirySHANGHAI T&W PHARMACEUTICAL CO., LTD.
A substitute for perfluorooctanoic acid, mainly used as a surfactant, dispersant, additive, etc Appearance:White solid or Colorless liquid Purity:99.3 % We will ship the goods in a timely manner as required We can provide relevant documents acc
Cas:951127-25-6
Min.Order:4 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryNanjing Fred Technology Co.,Ltd.
CPo3604-01 is one of the most competitive products in our company, we can supply it with good quality and best price. Appearance:White to off-white solid Storage:Room temperature, Keep in Dark Place. Package:Kilograms/MT Application:Pharmaceutical fi
Afine Chemicals Limited
Company Introduction 1. Established in 2005, with two independent business divisions: Fine chemicals division; Pharmaceutical division. 2. Main product: Optical brightener Textile auxiliary Dye stuff Pigments
Cas:951127-25-6
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShanghai Send Pharmaceutical Technology Co., Ltd.
low price high quality high purity good sevice stock Appearance:white Storage:Preserve In Well-Closed, Light-Resistant and Tight Containers. Store In Cool & Dry Place Package:1g,5g,10g...1kg,5kg...more Application:Pharmaceutical interme
Shanghai Massive Chemical Technology Co., Ltd.
Massive Chemical is certified with ISO9001 and ISO14001 manufacturer for this product. We will offer all documents as requirement for the materials which includes, Certificate of Analysis, Material Safety Data Sheet, and Method of Analysis and
Zibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:951127-25-6
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryHangzhou Lingrui Chemical Co.,Ltd.
Tert-butyl [(2R,3S)-5-oxo-2-(2,5-difluorophenyl)tetradihydro-2H-pyran-3-yl]carbamateAppearance:white or light yellow crystalline powder Storage:Store in a cool,dry place and keep away from direct strong light Package:according to customers' requireme
Cas:951127-25-6
Min.Order:1 Gram
Negotiable
Type:Other
inquiryZibo Dorne chemical technology co. LTD
Product Details Grade: pharmaceutical grade Purity:99%+ ProductionCapacity: 1000 Kilogram/Month Scope of use: For scientific research only(The product must be used legally) Our Advantage 1. Best quality with competitive price. 2. Quick shipping,
Xiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
EAST CHEMSOURCES LIMITED
factory?direct?saleAppearance:White Powder Storage:Store In Dry, Cool And Ventilated Place Package:25kg/drum, also according to the clients requirement Application:It is widely used as a thickener, emulsifier and stabilizer Transportation:By Sea Or B
Cas:951127-25-6
Min.Order:1 Kilogram
FOB Price: $18.0 / 20.0
Type:Trading Company
inquiryShandong Mopai Biotechnology Co., LTD
Shandong Mopai Biotechnology Co., LTD is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemicals. W
KAISA GROUP INC
1.Applied in food field.it can improve the immune system and prolong life. 2.Appliedin cosmetic field.it can improve the skin care. 3.Applied in pharmaceutical field.it can treat various dieases. 4.Our product quality assurance will make our customer
Cas:951127-25-6
Min.Order:1 Metric Ton
FOB Price: $1.5
Type:Trading Company
inquirySAGECHEM LIMITED
SAGECHEM is a chemical R&D, manufacturing and distribution company in China since 2009, including pharmaceutical intermediates, agrochemical, dyestuff intermediates, organosilicone, API and etc. We also offer a full range of services in custom synthe
Cas:951127-25-6
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquirySuzhou Health Chemicals Co., Ltd.
High quality,stable supply chain.Appearance:white/off-white or light yellow Storage:Store in cool and dry place, keep away from strong light and heat. Package:aluminum bottle,glass bottle,PTFE bottle,cardboard drum Application:This product can be use
Cas:951127-25-6
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryGolden Pharma Co., Limited
GOLDEN PHARMA CO.,LIMITED.is a professional pharmaceutical company,our team have more than 20years expereince in pharmaceutical production and sales. we are a professional technical enterprise specializing in the R & D, production,QA regulation
Cas:951127-25-6
Min.Order:1 Kilogram
Negotiable
Type:Other
inquirySenova Technology Company Limited
1.High quality 2.High purity 3.Best price 4.Best service 5.Professional manufacturer 6.Loyal and honest supplier 7.Fast response to customer within 6 hours Application:Pharmaceutical Intermediate
Cas:951127-25-6
Min.Order:0
Negotiable
Type:Trading Company
inquiryChemvon Biotechnology Co. Ltd.
Chemvon Biotechnology is one of the leading high-technology manufacturer in the field of pharmaceutical and fine chemical industry. From origins as a research group in technology service, chemvon has progressed into an integrated company with a k
Cas:951127-25-6
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryBluecrystal chem-union
We are a Union of chemistry in China, consists of chemists,engineers, laboratories,factories in China. We organize surplus capacity of R&D and production as well as custom synthesis for chemical products and chemical business project. We are supp
Cas:951127-25-6
Min.Order:1 Metric Ton
FOB Price: $1.0
Type:Trading Company
inquirySynthetic route

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water; ethyl acetate at 20℃; | 95% |
-
-
1172623-99-2
tert-butyl ((2R,3S)-2-(2,5-difluorophenyl)-5-hydroxytetrahydro-2H-pyran-3-yl)carbamate

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| With Dess-Martin periodane In dichloromethane at 0 - 30℃; for 4h; | 94.7% |
| With Dess-Martin periodane In dichloromethane at 0 - 20℃; for 4h; | 94.7% |
| With Dess-Martin periodane In dichloromethane at 0 - 4℃; for 4h; | 94.7% |

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| With zinc(II) chloride In ethanol at 0℃; Inert atmosphere; Large scale; | 93.5% |
-
-
951127-31-4
[(2R,3S)-5-methylene-2-(2,5-difluorophenyl)tetrahydro-2H-pyran-3-yl]carbamic acid tert-butyl ester

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| With sodium periodate; ruthenium(III) chloride trihydrate In dichloromethane; water; acetonitrile at 25℃; for 3h; | 84% |
| With sodium periodate; rhodium(III) chloride | |
| With sodium periodate; osmium(VIII) oxide In 1,4-dioxane; water at 0℃; for 2h; |

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| With bis-[(trifluoroacetoxy)iodo]benzene In water; acetonitrile at 20℃; for 1h; | 79% |
| With bis-[(trifluoroacetoxy)iodo]benzene In water; acetonitrile at 20℃; for 0.5h; | 79% |
| With bis-[(trifluoroacetoxy)iodo]benzene In water; acetonitrile at 20℃; for 1h; | 79% |
-
-
951127-32-5
[(2R,3S)-5-hydroxy-5-hydroxymethyl-2-(2,5-difluorophenyl)tetrahydro-2H-pyran-3-yl]carbamic acid tert-butyl ester

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| With pyridine; lead(IV) acetate In methanol for 0.333333h; | |
| With pyridine; lead(IV) acetate In methanol at 0℃; for 0.333333h; | |
| With pyridine; lead(IV) tetraacetate In methanol at 0℃; for 0.333333h; |
-
-
35730-09-7
2,5-difluorobenzoyl chloride

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: N-ethyl-N,N-diisopropylamine 2: sodium hydride 3: Heating 4: sodium tetrahydroborate 5: Chiralpak 1A / ethanol; hexane / Resolution of racemate 6: zinc; hydrogenchloride / water 7: ChiralCel AD 8: rhodium(III) chloride; sodium periodate View Scheme | |
| Multi-step reaction with 9 steps 1: N-ethyl-N,N-diisopropylamine 2: sodium hydride 3: Heating 4: sodium tetrahydroborate 5: 1,8-diazabicyclo[5.4.0]undec-7-ene / methanol 6: Chiralpak 1A / ethanol; hexane / Resolution of racemate 7: zinc; hydrogenchloride / water 8: ChiralCel AD 9: rhodium(III) chloride; sodium periodate View Scheme |

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: sodium hydride 2: Heating 3: sodium tetrahydroborate 4: Chiralpak 1A / ethanol; hexane / Resolution of racemate 5: zinc; hydrogenchloride / water 6: ChiralCel AD 7: rhodium(III) chloride; sodium periodate View Scheme | |
| Multi-step reaction with 8 steps 1: sodium hydride 2: Heating 3: sodium tetrahydroborate 4: 1,8-diazabicyclo[5.4.0]undec-7-ene / methanol 5: Chiralpak 1A / ethanol; hexane / Resolution of racemate 6: zinc; hydrogenchloride / water 7: ChiralCel AD 8: rhodium(III) chloride; sodium periodate View Scheme |
-
-
951127-28-9
6-(2,5-difluorophenyl)-3-methylidene-5-nitro-3,4-dihydro-2H-pyran

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: sodium tetrahydroborate 2: Chiralpak 1A / ethanol; hexane / Resolution of racemate 3: zinc; hydrogenchloride / water 4: ChiralCel AD 5: rhodium(III) chloride; sodium periodate View Scheme | |
| Multi-step reaction with 6 steps 1: sodium tetrahydroborate 2: 1,8-diazabicyclo[5.4.0]undec-7-ene / methanol 3: Chiralpak 1A / ethanol; hexane / Resolution of racemate 4: zinc; hydrogenchloride / water 5: ChiralCel AD 6: rhodium(III) chloride; sodium periodate View Scheme | |
| Multi-step reaction with 5 steps 1: sodium tetrahydroborate / methanol / 1 h / 0 - 5 °C 2: hydrogenchloride; zinc / water; ethanol / 1 h / 0 °C 3: methanol / 15 h / 25 °C 4: sodium carbonate / water; acetonitrile / 2 h / 0 - 30 °C 5: sodium periodate; ruthenium(III) chloride trihydrate / water; acetonitrile; dichloromethane / 3 h / 25 °C View Scheme |

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: Chiralpak 1A / ethanol; hexane / Resolution of racemate 2: zinc; hydrogenchloride / water 3: ChiralCel AD 4: rhodium(III) chloride; sodium periodate View Scheme | |
| Multi-step reaction with 4 steps 1: zinc; acetic acid / ethanol / 1 h 2: dichloromethane / 20 h / 20 °C 3: Chiralpak IA / Resolution of racemate 4: sodium periodate; osmium(VIII) oxide / 1,4-dioxane; water / 2 h / 0 °C View Scheme |

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 1,8-diazabicyclo[5.4.0]undec-7-ene / methanol 2: Chiralpak 1A / ethanol; hexane / Resolution of racemate 3: zinc; hydrogenchloride / water 4: ChiralCel AD 5: rhodium(III) chloride; sodium periodate View Scheme |
-
-
951127-29-0
(2R,3S)-5-methylidenyl-3-nitro-2-(2,5-difluorophenyl)tetrahydro-2H-pyran

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: zinc; hydrogenchloride / water 2: ChiralCel AD 3: rhodium(III) chloride; sodium periodate View Scheme | |
| Multi-step reaction with 4 steps 1.1: zinc; hydrogenchloride / ethanol; water / 3 h 2.1: dichloromethane / 20 °C 3.1: osmium(VIII) oxide / acetone; quinoline; tert-butyl alcohol / 0.17 h / 20 °C 3.2: 48 h / 20 °C 4.1: sodium periodate / tetrahydrofuran; water / 4 h View Scheme | |
| Multi-step reaction with 4 steps 1.1: zinc; hydrogenchloride / ethanol / 3 h 2.1: dichloromethane / 20 °C 3.1: osmium(VIII) oxide / acetone; water; tert-butyl alcohol / 0.17 h / 20 °C 3.2: 48 h / 20 °C 4.1: sodium periodate / tetrahydrofuran; water / 4 h View Scheme | |
| Multi-step reaction with 4 steps 1.1: zinc; hydrogenchloride / water; ethanol / 3 h 2.1: dichloromethane / 20 °C 3.1: osmium(VIII) oxide / water; acetone; tert-butyl alcohol / 0.17 h / 20 °C 3.2: 48 h / 20 °C 4.1: sodium iodate / water; tetrahydrofuran / 4 h View Scheme | |
| Multi-step reaction with 4 steps 1.1: zinc; hydrogenchloride / water; ethanol / 3 h 2.1: dichloromethane / 20 °C 3.1: osmium(VIII) oxide / water; acetone; tert-butyl alcohol / 0.17 h / 20 °C 3.2: 48 h / 20 °C 4.1: sodium periodate / water; tetrahydrofuran / 4 h View Scheme |
-
-
951127-30-3
(2R,3S)-2-(2,5-difluorophenyl)-5-methylenetetrahydro-2H-pyran-3-amine

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: ChiralCel AD 2: rhodium(III) chloride; sodium periodate View Scheme | |
| Multi-step reaction with 3 steps 1.1: dichloromethane / 20 °C 2.1: tert-butyl alcohol; osmium(VIII) oxide / acetone; water; tert-butyl alcohol / 0.17 h / 20 °C 2.2: 48 h / 20 °C 3.1: sodium periodate / tetrahydrofuran; water / 4 h View Scheme | |
| Multi-step reaction with 3 steps 1.1: dichloromethane / 20 °C 2.1: osmium(VIII) oxide / acetone; quinoline; tert-butyl alcohol / 0.17 h / 20 °C 2.2: 48 h / 20 °C 3.1: sodium periodate / tetrahydrofuran; water / 4 h View Scheme |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: ChiralCel AD 2: rhodium(III) chloride; sodium periodate View Scheme | |
| Multi-step reaction with 3 steps 1: dichloromethane / 20 h / 20 °C 2: Chiralpak IA / Resolution of racemate 3: sodium periodate; osmium(VIII) oxide / 1,4-dioxane; water / 2 h / 0 °C View Scheme | |
| Multi-step reaction with 7 steps 1.1: sodium hydroxide / tert-butyl methyl ether; water / 4 h / 20 °C / Inert atmosphere 2.1: 1,1'-carbonyldiimidazole / N,N-dimethyl-formamide / 15 - 25 °C / Inert atmosphere 2.2: 20 - 25 °C / Inert atmosphere 3.1: TurboGrignard / tetrahydrofuran; toluene / 2.5 h / -10 - -5 °C / Inert atmosphere 3.2: 2.5 h / -10 - 20 °C / Inert atmosphere 4.1: 1,4-diaza-bicyclo[2.2.2]octane; formic acid; [(R,R)-N-(2-amino-1,2-diphenylethyl)pentafluorobenzenesulfonamide]chloride(p-cymene)ruthenium (II) / tetrahydrofuran / Inert atmosphere 5.1: tert-butylammonium hexafluorophosphate(V); 1-hydroxy-pyrrolidine-2,5-dione; sodium hydrogencarbonate; chlorocyclopentadienylbis(triphenylphosphine)ruthenium(II) ethanol; triphenylphosphine / N,N-dimethyl-formamide; n-heptane / 16 h / 80 °C / Inert atmosphere 6.1: dimethylsulfide borane complex / tert-butyl methyl ether / 0 - 5 °C / Inert atmosphere 6.2: 15 - 25 °C / Inert atmosphere 7.1: acetic acid; ruthenium(III) chloride trihydrate; sodium bromate / water; acetonitrile / 0 - 2 °C / Inert atmosphere View Scheme |
-
-
951127-27-8
1-(2,5-difluorophenyl)-2-nitroethanone

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: Heating 2: sodium tetrahydroborate 3: Chiralpak 1A / ethanol; hexane / Resolution of racemate 4: zinc; hydrogenchloride / water 5: ChiralCel AD 6: rhodium(III) chloride; sodium periodate View Scheme | |
| Multi-step reaction with 7 steps 1: Heating 2: sodium tetrahydroborate 3: 1,8-diazabicyclo[5.4.0]undec-7-ene / methanol 4: Chiralpak 1A / ethanol; hexane / Resolution of racemate 5: zinc; hydrogenchloride / water 6: ChiralCel AD 7: rhodium(III) chloride; sodium periodate View Scheme | |
| Multi-step reaction with 6 steps 1: caesium carbonate / N,N-dimethyl acetamide / 4 h / 25 - 30 °C 2: sodium tetrahydroborate / methanol / 1 h / 0 - 5 °C 3: hydrogenchloride; zinc / water; ethanol / 1 h / 0 °C 4: methanol / 15 h / 25 °C 5: sodium carbonate / water; acetonitrile / 2 h / 0 - 30 °C 6: sodium periodate; ruthenium(III) chloride trihydrate / water; acetonitrile; dichloromethane / 3 h / 25 °C View Scheme |
-
-
1172623-98-1
tert-butyl ((2R,3S)-2-(2,5-difluorophenyl)-3,4-dihydro-2H-pyran-3-yl)carbamate

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: dimethylsulfide borane complex / tert-butyl methyl ether / 2 h / 0 - 10 °C 1.2: 7 h / 0 - 20 °C 2.1: sodiumtetraborate tetrahydrate; acetic acid; RuC13•3H20 / acetonitrile; water / 7.5 h / 0 °C / Inert atmosphere 2.2: 5.5 h / 0 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: dimethylsulfide borane complex / tert-butyl methyl ether / 0 - 5 °C / Inert atmosphere 1.2: 15 - 25 °C / Inert atmosphere 2.1: acetic acid; ruthenium(III) chloride trihydrate; sodium bromate / water; acetonitrile / 0 - 2 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1.1: dimethylsulfide borane complex / tert-butyl methyl ether / 0 - 5 °C / Large scale 1.2: 0 - 20 °C / Large scale 2.1: acetic acid; ruthenium(III) chloride trihydrate; sodium bromate / water; acetonitrile / 15 h / 0 - 20 °C / Inert atmosphere; Large scale View Scheme |
-
-
1172623-99-2
tert-butyl ((2R,3S)-2-(2,5-difluorophenyl)-5-hydroxytetrahydro-2H-pyran-3-yl)carbamate

-
-
67-63-0
isopropyl alcohol

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl [(2R,3S)-2-(2,5-difluorophenyl)-5-hydroxytetrahydro-2H-pyran-3-yl]carbamate With RuC13•3H20; sodiumtetraborate tetrahydrate; acetic acid In water; acetonitrile at 0℃; for 7.5h; Inert atmosphere; Stage #2: isopropyl alcohol In water; acetonitrile at 0℃; for 5.5h; |
-
-
1172623-95-8
(±)-tert-butyl (1-(methoxy(methyl)amino)-1-oxopent-4-yn-2-yl)carbamate

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: isopropylmagnesium chloride / dichloromethane; tetrahydrofuran / 3 h 1.2: 12 h / -20 - 20 °C 2.1: 1,4-diaza-bicyclo[2.2.2]octane; Noyori's catalyst; formic acid / tert-butyl methyl ether; tetrahydrofuran / 45 °C / Inert atmosphere 3.1: tert-butylammonium hexafluorophosphate(V); sodium hydrogencarbonate; chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium (II); triphenylphosphine / tert-butyl methyl ether; N,N-dimethyl-formamide / 75 - 85 °C / Inert atmosphere 4.1: dimethylsulfide borane complex / tert-butyl methyl ether / 2 h / 0 - 10 °C 4.2: 7 h / 0 - 20 °C 5.1: sodiumtetraborate tetrahydrate; acetic acid; RuC13•3H20 / acetonitrile; water / 7.5 h / 0 °C / Inert atmosphere 5.2: 5.5 h / 0 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: TurboGrignard / tetrahydrofuran; toluene / 2.5 h / -10 - -5 °C / Inert atmosphere 1.2: 2.5 h / -10 - 20 °C / Inert atmosphere 2.1: 1,4-diaza-bicyclo[2.2.2]octane; formic acid; [(R,R)-N-(2-amino-1,2-diphenylethyl)pentafluorobenzenesulfonamide]chloride(p-cymene)ruthenium (II) / tetrahydrofuran / Inert atmosphere 3.1: tert-butylammonium hexafluorophosphate(V); 1-hydroxy-pyrrolidine-2,5-dione; sodium hydrogencarbonate; chlorocyclopentadienylbis(triphenylphosphine)ruthenium(II) ethanol; triphenylphosphine / N,N-dimethyl-formamide; n-heptane / 16 h / 80 °C / Inert atmosphere 4.1: dimethylsulfide borane complex / tert-butyl methyl ether / 0 - 5 °C / Inert atmosphere 4.2: 15 - 25 °C / Inert atmosphere 5.1: acetic acid; ruthenium(III) chloride trihydrate; sodium bromate / water; acetonitrile / 0 - 2 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1.1: TurboGrignard / tetrahydrofuran; toluene / 2.5 h / -10 - -5 °C / Inert atmosphere 1.2: 2.5 h / -10 - 20 °C / Inert atmosphere 2.1: 1,4-diaza-bicyclo[2.2.2]octane; ((R,R)-Ts-DENEB)RuCl; formic acid / tetrahydrofuran / 10 - 35 °C / Inert atmosphere 3.1: tert-butylammonium hexafluorophosphate(V); 1-hydroxy-pyrrolidine-2,5-dione; sodium hydrogencarbonate; chlorocyclopentadienylbis(triphenylphosphine)ruthenium(II) ethanol; triphenylphosphine / N,N-dimethyl-formamide; n-heptane / 16 h / 80 °C / Inert atmosphere 4.1: dimethylsulfide borane complex / tert-butyl methyl ether / 0 - 5 °C / Inert atmosphere 4.2: 15 - 25 °C / Inert atmosphere 5.1: acetic acid; ruthenium(III) chloride trihydrate; sodium bromate / water; acetonitrile / 0 - 2 °C / Inert atmosphere View Scheme |
-
-
1172623-96-9
(±)-tert-butyl (1-(2,5-difluorophenyl)-1-oxopent-4-yn-2-yl)carbamate

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 1,4-diaza-bicyclo[2.2.2]octane; Noyori's catalyst; formic acid / tert-butyl methyl ether; tetrahydrofuran / 45 °C / Inert atmosphere 2.1: tert-butylammonium hexafluorophosphate(V); sodium hydrogencarbonate; chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium (II); triphenylphosphine / tert-butyl methyl ether; N,N-dimethyl-formamide / 75 - 85 °C / Inert atmosphere 3.1: dimethylsulfide borane complex / tert-butyl methyl ether / 2 h / 0 - 10 °C 3.2: 7 h / 0 - 20 °C 4.1: sodiumtetraborate tetrahydrate; acetic acid; RuC13•3H20 / acetonitrile; water / 7.5 h / 0 °C / Inert atmosphere 4.2: 5.5 h / 0 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: 1,4-diaza-bicyclo[2.2.2]octane; formic acid; [(R,R)-N-(2-amino-1,2-diphenylethyl)pentafluorobenzenesulfonamide]chloride(p-cymene)ruthenium (II) / tetrahydrofuran / Inert atmosphere 2.1: tert-butylammonium hexafluorophosphate(V); 1-hydroxy-pyrrolidine-2,5-dione; sodium hydrogencarbonate; chlorocyclopentadienylbis(triphenylphosphine)ruthenium(II) ethanol; triphenylphosphine / N,N-dimethyl-formamide; n-heptane / 16 h / 80 °C / Inert atmosphere 3.1: dimethylsulfide borane complex / tert-butyl methyl ether / 0 - 5 °C / Inert atmosphere 3.2: 15 - 25 °C / Inert atmosphere 4.1: acetic acid; ruthenium(III) chloride trihydrate; sodium bromate / water; acetonitrile / 0 - 2 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1.1: 1,4-diaza-bicyclo[2.2.2]octane; ((R,R)-Ts-DENEB)RuCl; formic acid / tetrahydrofuran / 10 - 35 °C / Inert atmosphere 2.1: tert-butylammonium hexafluorophosphate(V); 1-hydroxy-pyrrolidine-2,5-dione; sodium hydrogencarbonate; chlorocyclopentadienylbis(triphenylphosphine)ruthenium(II) ethanol; triphenylphosphine / N,N-dimethyl-formamide; n-heptane / 16 h / 80 °C / Inert atmosphere 3.1: dimethylsulfide borane complex / tert-butyl methyl ether / 0 - 5 °C / Inert atmosphere 3.2: 15 - 25 °C / Inert atmosphere 4.1: acetic acid; ruthenium(III) chloride trihydrate; sodium bromate / water; acetonitrile / 0 - 2 °C / Inert atmosphere View Scheme |

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: tert-butylammonium hexafluorophosphate(V); sodium hydrogencarbonate; chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium (II); triphenylphosphine / tert-butyl methyl ether; N,N-dimethyl-formamide / 75 - 85 °C / Inert atmosphere 2.1: dimethylsulfide borane complex / tert-butyl methyl ether / 2 h / 0 - 10 °C 2.2: 7 h / 0 - 20 °C 3.1: sodiumtetraborate tetrahydrate; acetic acid; RuC13•3H20 / acetonitrile; water / 7.5 h / 0 °C / Inert atmosphere 3.2: 5.5 h / 0 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: 1-hydroxy-pyrrolidine-2,5-dione; tert-butylammonium hexafluorophosphate(V); sodium hydrogencarbonate / N,N-dimethyl-formamide / Inert atmosphere; Large scale 1.2: 75 - 85 °C / Inert atmosphere; Large scale 2.1: dimethylsulfide borane complex / tert-butyl methyl ether / 0 - 5 °C / Large scale 2.2: 0 - 20 °C / Large scale 3.1: acetic acid; ruthenium(III) chloride trihydrate; sodium bromate / water; acetonitrile / 15 h / 0 - 20 °C / Inert atmosphere; Large scale View Scheme |

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: hydrogenchloride; zinc / water; ethanol / 1 h / 0 °C 2: methanol / 15 h / 25 °C 3: sodium carbonate / water; acetonitrile / 2 h / 0 - 30 °C 4: sodium periodate; ruthenium(III) chloride trihydrate / water; acetonitrile; dichloromethane / 3 h / 25 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: ChiralCel OD / Resolution of racemate 2.1: zinc; hydrogenchloride / ethanol; water / 3 h 3.1: dichloromethane / 20 °C 4.1: osmium(VIII) oxide / acetone; quinoline; tert-butyl alcohol / 0.17 h / 20 °C 4.2: 48 h / 20 °C 5.1: sodium periodate / tetrahydrofuran; water / 4 h View Scheme |

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: isopropyl alcohol / 90 °C 2: hydrogenchloride; zinc / water; ethanol / 1 h / 0 °C 3: methanol / 15 h / 25 °C 4: sodium carbonate / water; acetonitrile / 2 h / 0 - 30 °C 5: sodium periodate; ruthenium(III) chloride trihydrate / water; acetonitrile; dichloromethane / 3 h / 25 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / tetrahydrofuran / 0.5 h / 20 °C 2.1: zinc; hydrogenchloride / water; ethanol / 3 h 3.1: dichloromethane / 20 °C 4.1: osmium(VIII) oxide / water; acetone; tert-butyl alcohol / 0.17 h / 20 °C 4.2: 48 h / 20 °C 5.1: sodium iodate / water; tetrahydrofuran / 4 h View Scheme | |
| Multi-step reaction with 5 steps 1.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / tetrahydrofuran / 0.5 h / 20 °C 2.1: zinc; hydrogenchloride / water; ethanol / 3 h 3.1: dichloromethane / 20 °C 4.1: osmium(VIII) oxide / water; acetone; tert-butyl alcohol / 0.17 h / 20 °C 4.2: 48 h / 20 °C 5.1: sodium periodate / water; tetrahydrofuran / 4 h View Scheme |

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: methanol / 15 h / 25 °C 2: sodium carbonate / water; acetonitrile / 2 h / 0 - 30 °C 3: sodium periodate; ruthenium(III) chloride trihydrate / water; acetonitrile; dichloromethane / 3 h / 25 °C View Scheme |

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium carbonate / water; acetonitrile / 2 h / 0 - 30 °C 2: sodium periodate; ruthenium(III) chloride trihydrate / water; acetonitrile; dichloromethane / 3 h / 25 °C View Scheme |
-
-
2646-90-4
2,5-difluorobenzaldehyde

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: sodium hydroxide / methanol; water / 0.5 h / 0 °C 2: Jones reagent / acetone / 0 - 10 °C 3: caesium carbonate / N,N-dimethyl acetamide / 4 h / 25 - 30 °C 4: sodium tetrahydroborate / methanol / 1 h / 0 - 5 °C 5: hydrogenchloride; zinc / water; ethanol / 1 h / 0 °C 6: methanol / 15 h / 25 °C 7: sodium carbonate / water; acetonitrile / 2 h / 0 - 30 °C 8: sodium periodate; ruthenium(III) chloride trihydrate / water; acetonitrile; dichloromethane / 3 h / 25 °C View Scheme | |
| Multi-step reaction with 8 steps 1: sodium hydroxide / methanol / 1 h / 5 °C 2: Dess-Martin periodane / dichloromethane / 2.5 h / 10 °C 3: N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 2 h / 60 °C 4: silica gel; sodium tetrahydroborate / chloroform; isopropyl alcohol / 2 h / 20 °C 5: zinc; acetic acid / ethanol / 1 h 6: dichloromethane / 20 h / 20 °C 7: Chiralpak IA / Resolution of racemate 8: sodium periodate; osmium(VIII) oxide / 1,4-dioxane; water / 2 h / 0 °C View Scheme | |
| Multi-step reaction with 10 steps 1.1: sodium hydroxide / methanol / 5 °C 2.1: Dess-Martin periodane / dichloromethane / 24.5 h / 10 °C 3.1: N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 2 h / 20 °C 4.1: silica gel; sodium tetrahydroborate / chloroform; isopropyl alcohol / 20 °C 5.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / tetrahydrofuran / 0.5 h / 20 °C 6.1: ChiralCel OD / Resolution of racemate 7.1: zinc; hydrogenchloride / ethanol; water / 3 h 8.1: dichloromethane / 20 °C 9.1: tert-butyl alcohol; osmium(VIII) oxide / acetone; water; tert-butyl alcohol / 0.17 h / 20 °C 9.2: 48 h / 20 °C 10.1: sodium periodate / tetrahydrofuran; water / 4 h View Scheme |
-
-
951127-26-7
1-(2,5-difluorophenyl)-2-nitroethanol

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: Jones reagent / acetone / 0 - 10 °C 2: caesium carbonate / N,N-dimethyl acetamide / 4 h / 25 - 30 °C 3: sodium tetrahydroborate / methanol / 1 h / 0 - 5 °C 4: hydrogenchloride; zinc / water; ethanol / 1 h / 0 °C 5: methanol / 15 h / 25 °C 6: sodium carbonate / water; acetonitrile / 2 h / 0 - 30 °C 7: sodium periodate; ruthenium(III) chloride trihydrate / water; acetonitrile; dichloromethane / 3 h / 25 °C View Scheme | |
| Multi-step reaction with 7 steps 1: Dess-Martin periodane / dichloromethane / 2.5 h / 10 °C 2: N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 2 h / 60 °C 3: silica gel; sodium tetrahydroborate / chloroform; isopropyl alcohol / 2 h / 20 °C 4: zinc; acetic acid / ethanol / 1 h 5: dichloromethane / 20 h / 20 °C 6: Chiralpak IA / Resolution of racemate 7: sodium periodate; osmium(VIII) oxide / 1,4-dioxane; water / 2 h / 0 °C View Scheme | |
| Multi-step reaction with 8 steps 1.1: Dess-Martin periodane / dichloromethane / 24.5 h / 10 °C 2.1: N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 2 h / 20 °C 3.1: silica gel; sodium tetrahydroborate / chloroform; isopropyl alcohol / 20 °C 4.1: ChiralCel OD / Resolution of racemate 5.1: zinc; hydrogenchloride / ethanol; water / 3 h 6.1: dichloromethane / 20 °C 7.1: tert-butyl alcohol; osmium(VIII) oxide / acetone; water; tert-butyl alcohol / 0.17 h / 20 °C 7.2: 48 h / 20 °C 8.1: sodium periodate / tetrahydrofuran; water / 4 h View Scheme |

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: dichloromethane / 20 h / 20 °C 2: Chiralpak IA / Resolution of racemate 3: sodium periodate; osmium(VIII) oxide / 1,4-dioxane; water / 2 h / 0 °C View Scheme |

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: dimethylsulfide borane complex / tert-butyl methyl ether; toluene / 3 h / -7 - 15 °C 2.1: acetic acid; ruthenium trichloride; sodium bromate / water; acetonitrile / 0 °C 2.2: 5 h / 0 °C View Scheme |
-
-
50428-03-0
D,L-propargylglycine

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: sodium hydroxide / tert-butyl methyl ether; water / 4 h / 20 °C / Inert atmosphere 2.1: 1,1'-carbonyldiimidazole / N,N-dimethyl-formamide / 15 - 25 °C / Inert atmosphere 2.2: 20 - 25 °C / Inert atmosphere 3.1: TurboGrignard / tetrahydrofuran; toluene / 2.5 h / -10 - -5 °C / Inert atmosphere 3.2: 2.5 h / -10 - 20 °C / Inert atmosphere 4.1: 1,4-diaza-bicyclo[2.2.2]octane; formic acid; [(R,R)-N-(2-amino-1,2-diphenylethyl)pentafluorobenzenesulfonamide]chloride(p-cymene)ruthenium (II) / tetrahydrofuran / Inert atmosphere 5.1: tert-butylammonium hexafluorophosphate(V); 1-hydroxy-pyrrolidine-2,5-dione; sodium hydrogencarbonate; chlorocyclopentadienylbis(triphenylphosphine)ruthenium(II) ethanol; triphenylphosphine / N,N-dimethyl-formamide; n-heptane / 16 h / 80 °C / Inert atmosphere 6.1: dimethylsulfide borane complex / tert-butyl methyl ether / 0 - 5 °C / Inert atmosphere 6.2: 15 - 25 °C / Inert atmosphere 7.1: acetic acid; ruthenium(III) chloride trihydrate; sodium bromate / water; acetonitrile / 0 - 2 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 7 steps 1.1: sodium hydroxide / tert-butyl methyl ether; water / 4 h / 20 °C / Inert atmosphere 2.1: 1,1'-carbonyldiimidazole / N,N-dimethyl-formamide / 15 - 25 °C / Inert atmosphere 2.2: 20 - 25 °C / Inert atmosphere 3.1: TurboGrignard / tetrahydrofuran; toluene / 2.5 h / -10 - -5 °C / Inert atmosphere 3.2: 2.5 h / -10 - 20 °C / Inert atmosphere 4.1: 1,4-diaza-bicyclo[2.2.2]octane; ((R,R)-Ts-DENEB)RuCl; formic acid / tetrahydrofuran / 10 - 35 °C / Inert atmosphere 5.1: tert-butylammonium hexafluorophosphate(V); 1-hydroxy-pyrrolidine-2,5-dione; sodium hydrogencarbonate; chlorocyclopentadienylbis(triphenylphosphine)ruthenium(II) ethanol; triphenylphosphine / N,N-dimethyl-formamide; n-heptane / 16 h / 80 °C / Inert atmosphere 6.1: dimethylsulfide borane complex / tert-butyl methyl ether / 0 - 5 °C / Inert atmosphere 6.2: 15 - 25 °C / Inert atmosphere 7.1: acetic acid; ruthenium(III) chloride trihydrate; sodium bromate / water; acetonitrile / 0 - 2 °C / Inert atmosphere View Scheme |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester; ethyl (S)-1-(methylsulfonyl)-1,4,5,6-tetrahydropyrrolo[3,4-c]pyrazole-4-carboxylate With benzenesulfonic acid In N,N-dimethyl acetamide at 20℃; for 1h; Stage #2: With sodium tris(acetoxy)borohydride In N,N-dimethyl acetamide at 20℃; for 16h; | 100% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride In N,N-dimethyl acetamide at 0 - 20℃; for 8h; Inert atmosphere; | 99% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

-
-
104-94-9
4-methoxy-aniline

| Conditions | Yield |
|---|---|
| Stage #1: N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester; 4-methoxy-aniline In trifluoroacetic acid at 0℃; for 1h; Stage #2: With N,N-dimethyl acetamide; sodium tris(acetoxy)borohydride at 0 - 2℃; for 5h; pH=9; | 98% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

-
-
870-46-2
t-butoxycarbonylhydrazine

| Conditions | Yield |
|---|---|
| Stage #1: N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester; t-butoxycarbonylhydrazine With sodium cyanoborohydride at 25℃; for 0.166667h; Stage #2: With sodium cyanoborohydride at 25℃; for 16h; | 94.9% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

-
-
1280210-80-1
2-(methylsulfonyl)-2,4,5,6-tetrahydropyrrolo[3,4-c]pyrazol-5-ium benzenesulfonate

-
-
1226781-87-8
tert-butyl ((2R,3S,5R)-2-(2,5-difluorophenyl)-5-(2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl)tetrahydro-2H-pyran-3-yl)carbamate

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride In N,N-dimethyl acetamide at -10℃; for 2h; | 94% |
| With sodium tris(acetoxy)borohydride; acetic acid In N,N-dimethyl acetamide at -15℃; Reagent/catalyst; | 93.2% |
| With sodium tris(acetoxy)borohydride; orthoformic acid triethyl ester In N,N-dimethyl acetamide at 5 - 50℃; for 12h; Solvent; Temperature; Inert atmosphere; Large scale; | 82.5% |
| With sodium tris(acetoxy)borohydride In N,N-dimethyl acetamide at 0 - 20℃; for 7.66667h; Large scale; | |
| With sodium tris(acetoxy)borohydride In N,N-dimethyl acetamide at 0 - 20℃; for 7.66667h; Large scale; |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester; 3-bromo-6,7-dihydro-5H-pyrrolo[3,4-b]pyridine hydrochloride With N,N`-dimethylethylenediamine In N,N-dimethyl acetamide at 20℃; for 0.25h; Stage #2: With acetic acid In N,N-dimethyl acetamide for 0.25h; Stage #3: With sodium tris(acetoxy)borohydride In N,N-dimethyl acetamide at -10 - 20℃; | 93.82% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester; C7H8O3S*C10H11N3O2S2 In N,N-dimethyl acetamide at 20℃; for 0.166667h; Stage #2: With sodium tris(acetoxy)borohydride at 0 - 20℃; for 12h; Inert atmosphere; | 92% |
-
-
40499-83-0
pyrrolidin-3-ol

-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: pyrrolidin-3-ol; N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester In methanol at 20℃; for 1h; Stage #2: With nido-decaborane In methanol at 20℃; | 90% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride; acetic acid; triethylamine In N,N-dimethyl acetamide at 20℃; for 2.5h; | 88% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester; 1-ethyl-1,4,5,6-tetrahydropyrrolo[3,4-d]imidazole-2-carbonitrile benzenesulfonate In N,N-dimethyl acetamide at 20℃; for 1h; Stage #2: With sodium tris(acetoxy)borohydride In N,N-dimethyl acetamide at 0 - 20℃; for 2h; | 87% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

-
-
540-63-6
ethane-1,2-dithiol

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In dichloromethane at 20℃; for 1h; Inert atmosphere; Sealed tube; | 86% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

-
-
540-63-6
ethane-1,2-dithiol

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In dichloromethane at 20℃; Inert atmosphere; Sealed tube; | 86% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester


| Conditions | Yield |
|---|---|
| Stage #1: N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester; 1-(difluoromethyl)-2-(1-methyl-1H-tetrazol-5-yl)-1,4,5,6-tetrahydropyrrolo[3,4-d]imidazole benzenesulfonate In N,N-dimethyl acetamide at 20℃; for 0.5h; Stage #2: With sodium tris(acetoxy)borohydride In N,N-dimethyl acetamide at 0 - 20℃; for 2.5h; | 85% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

-
-
919346-89-7
5-bromo-2,3-dihydro-1H-isoindole hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester; 5-bromo-2,3-dihydro-1H-isoindole hydrochloride With triethylamine In N,N-dimethyl acetamide at 10℃; for 0.25h; Stage #2: With acetic acid In N,N-dimethyl acetamide for 1h; Stage #3: With sodium tris(acetoxy)borohydride In N,N-dimethyl acetamide at 0 - 10℃; for 16h; | 84.4% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester; 1-ethyl-N-methoxy-N-methyl-1,4,5,6-tetrahydropyrrolo[3,4-d]imidazole-2-carboxamide benzenesulfonate In N,N-dimethyl acetamide at 20℃; for 0.5h; Stage #2: With sodium tris(acetoxy)borohydride In N,N-dimethyl acetamide at 20℃; for 2.5h; | 82% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester; 7-methanesulfonyl-2,7-diazaspiro[3.4]octane hydrochloride In methanol at 0℃; for 6h; Inert atmosphere; Stage #2: With diborane In methanol at 20℃; for 12h; Inert atmosphere; | 81% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester


-
-
1226781-87-8
tert-butyl ((2R,3S,5R)-2-(2,5-difluorophenyl)-5-(2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl)tetrahydro-2H-pyran-3-yl)carbamate

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride In N,N-dimethyl acetamide at -10℃; | 80% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester; C10H13N5*2C7H8O3S In N,N-dimethyl acetamide at 20℃; for 1h; Stage #2: With sodium tris(acetoxy)borohydride In N,N-dimethyl acetamide at 20℃; for 2h; | 80% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester; 2-((methylsulfonyl)methyl)-2,4,5,6-tetrahydropyrrolo[3,4-c]pyrazole benzenesulfonate In N,N-dimethyl acetamide at 20℃; for 1h; Stage #2: With sodium tris(acetoxy)borohydride In N,N-dimethyl acetamide at 20℃; for 16h; | 79.7% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester; 1-ethyl-2-(methylsulfonyl)-1,4,5,6-tetrahydropyrrolo[3,4-d]imidazole benzenesulfonate In N,N-dimethyl acetamide at 20℃; for 1h; Stage #2: With sodium tris(acetoxy)borohydride In N,N-dimethyl acetamide at 0 - 20℃; for 16h; | 79% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester; C7H8O3S*C9H9N3O2S2 In N,N-dimethyl acetamide at 20℃; for 0.166667h; Stage #2: With sodium tris(acetoxy)borohydride at 0 - 20℃; for 12h; Inert atmosphere; | 79% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester; C7H12N4O2S With nido-decaborane In methanol at 20℃; Stage #2: With trifluoroacetic acid In dichloromethane at 20℃; for 2h; | 78% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

-
-
870-46-2
t-butoxycarbonylhydrazine

| Conditions | Yield |
|---|---|
| Stage #1: N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester; t-butoxycarbonylhydrazine With acetic acid at 25℃; for 0.166667h; Stage #2: With sodium cyanoborohydride at 25℃; for 3h; | 76.9% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride In N,N-dimethyl acetamide at 20℃; for 3h; | 72% |
-
-
951127-25-6
N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: N-[(2R,3S)-2-(2,5-difluorophenyl)tetrahydro-5-oxo-2H-pyran-3-yl]carbamic acid 1,1-dimethylethyl ester; 2,3,4,5,6,7-hexahydropyrrolo[3',4':3,4]pyrazolo[1,5-b][1,2]thiazine-1,1-dioxide p-toluenesulfonate With triethylamine In N,N-dimethyl acetamide at 20℃; for 3h; Stage #2: With sodium tris(acetoxy)borohydride In N,N-dimethyl acetamide at 20℃; Cooling with ice; | 71% |
Related products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View