-
Name
(2S,3S,5S)-5-Amino-2-(benzylamino)-1,6-diphenylhexan-3-ol
- EINECS
- CAS No. 156732-15-9
- Article Data16
- CAS DataBase
- Density 1.125 g/cm3
- Solubility
- Melting Point
- Formula C32H36N2O
- Boiling Point 642.878 °C at 760 mmHg
- Molecular Weight 464.651
- Flash Point 342.601 °C
- Transport Information
- Appearance Colourless thick oil
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Benzenebutanol,g-amino-a-[1-[bis(phenylmethyl)amino]-2-phenylethyl]-,[aS-[aR*(R*),gR*]]-;(2S,3S,5S)-5-Amino-2-(dibenzylamino)-3-hydroxy-1,6-diphenylhexane;
- PSA 49.49000
- LogP 6.32140
Synthetic route

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Stage #1: (5S)-2-amino-5-dibenzylamino-4-oxo-1,6-diphenylhex-2-ene With sodium tetrahydroborate; methanesulfonic acid at 6℃; for 1.5h; Stage #2: With sodium tetrahydroborate; trifluoroacetic acid at 6℃; for 1.5h; | 93% |
| Stage #1: (5S)-2-amino-5-dibenzylamino-4-oxo-1,6-diphenylhex-2-ene With sodium tetrahydroborate; methanesulfonic acid In tetrahydrofuran; isopropyl alcohol at 0 - 10℃; Stage #2: With trifluoroacetic acid In tetrahydrofuran; isopropyl alcohol at 0 - 15℃; for 4.75h; | 55% |
| Stage #1: (5S)-2-amino-5-dibenzylamino-4-oxo-1,6-diphenylhex-2-ene With sodium tetrahydroborate; methanesulfonic acid In 1,2-dimethoxyethane; isopropyl alcohol at -5℃; for 12h; Stage #2: With triethanolamine In 1,2-dimethoxyethane; isopropyl alcohol at -5℃; for 0.5h; Stage #3: In 1,2-dimethoxyethane; N,N-dimethyl acetamide; isopropyl alcohol at 0℃; for 2h; | 40% |

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Stage #1: (S,E)-5-amino-2-(dibenzylamino)-1,6-diphenylhex-4-en-3-one With sodium tetrahydroborate; methanesulfonic acid In diethylene glycol dimethyl ether; isopropyl alcohol at 0 - 5℃; for 12h; Stage #2: With triethanolamine In diethylene glycol dimethyl ether; isopropyl alcohol at 0 - 5℃; for 0.5h; Stage #3: With sodium tetrahydroborate In diethylene glycol dimethyl ether; N,N-dimethyl acetamide; isopropyl alcohol at 15℃; for 3h; | 65% |
-
-
7440-06-4
platinum

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| With methanesulfonic acid; hydrogen In ethanol | |
| With methanesulfonic acid; hydrogen In ethanol |

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-5S-dibenzylamino-4-oxo-1,6-diphenylhexane With sodium tetrahydroborate; methanesulfonic acid In 1,2-dimethoxyethane; isopropyl alcohol at 15 - 25℃; for 1h; Stage #2: With triethanolamine In ISOPROPYLAMIDE; water at 10 - 30℃; for 0.5h; | |
| Stage #1: 2-amino-5S-dibenzylamino-4-oxo-1,6-diphenylhexane With sodium tetrahydroborate; methanesulfonic acid In 1,2-dimethoxyethane; isopropyl alcohol at 20℃; for 6h; Stage #2: With triethanolamine In ISOPROPYLAMIDE at 15℃; for 1h; |
-
-
220887-32-1
(5S)-2-Amino-5-(N,N-dibenzylamino)-4-oxo-1,6-diphenylhex-2-ene

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Stage #1: (5S)-2-Amino-5-(N,N-dibenzylamino)-4-oxo-1,6-diphenylhex-2-ene With sodium tetrahydroborate; methanesulfonic acid In Dimethyl ether; isopropyl alcohol at 0 - 10℃; for 12.5h; Stage #2: With triethanolamine In Dimethyl ether; ISOPROPYLAMIDE; isopropyl alcohol at 0 - 20℃; for 2.91667h; Stage #3: With water In Dimethyl ether; ISOPROPYLAMIDE; isopropyl alcohol at 10 - 20℃; |

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| With sodium borohydrid; methanesulfonic acid In 1,2-dimethoxyethane; tert-butyl methyl ether; ISOPROPYLAMIDE; isopropyl alcohol |
-
-
172526-43-1
(5S,1'S)-3-phenylmethyl-5-(1'-N,N-dibenzylamino-2'-phenylethyl)-2-isoxazoline

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| With sodium hydroxide In di-isopropyl ether; water | |
| Pt-on-carbon In ethanol; ammonia |

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water; ethyl acetate at 25 - 30℃; for 0.25h; |
-
-
156732-14-8
(2S,5S)-5-amino-2-dibenzylamino-3-oxo-1,6-diphenylhexane

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Stage #1: (2S,5S)-5-amino-2-dibenzylamino-3-oxo-1,6-diphenylhexane With sodium tetrahydroborate; trifluoroacetic acid In 1,2-dimethoxyethane at -10 - 20℃; for 0.5h; Stage #2: With sodium hydroxide In 1,2-dimethoxyethane; water at 5 - 10℃; |

-
D
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Stage #1: (5S)-2-amino-5-dibenzylamino-4-oxo-1,6-diphenylhex-2-ene With sodium tetrahydroborate; methanesulfonic acid; isopropyl alcohol In 1,2-dimethoxyethane at 0 - 10℃; for 12h; Stage #2: With triethanolamine In 1,2-dimethoxyethane at 5℃; for 0.5h; Stage #3: With sodium tetrahydroborate In 1,2-dimethoxyethane; N,N-dimethyl acetamide at 10 - 20℃; for 2h; Reagent/catalyst; Solvent; diastereoselective reaction; | A n/a B n/a C n/a D n/a |

-
-
63-91-2
L-phenylalanine

-
D
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: potassium carbonate; potassium hydroxide / water / 5 h / Reflux 2.1: sodium amide / acetonitrile; tert-butyl methyl ether / 2 h / 0 - 5 °C / Large scale 2.2: 2 h / 25 °C / Large scale 3.1: sodium tetrahydroborate; methanesulfonic acid; isopropyl alcohol / 1,2-dimethoxyethane / 12 h / 0 - 10 °C 3.2: 0.5 h / 5 °C 3.3: 2 h / 10 - 20 °C View Scheme |

-
-
111138-83-1
N,N-dibenzyl-L-phenylalanine benzyl ester

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium amide / tetrahydrofuran / 0.5 h / -45 °C / Inert atmosphere 1.2: 3 h / -45 °C / Inert atmosphere 2.1: tetrahydrofuran / 0 - 20 °C 3.1: sodium tetrahydroborate; methanesulfonic acid / tetrahydrofuran; isopropyl alcohol / 0 - 10 °C 3.2: 4.75 h / 0 - 15 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium amide 2.1: 10 h / 5 °C 3.1: sodium tetrahydroborate; methanesulfonic acid / 1.5 h / 6 °C 3.2: 1.5 h / 6 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: sodium amide / tert-butyl methyl ether / 2 h / -5 - 0 °C 1.2: 2 h / 20 °C 1.3: 0 °C 2.1: sodium tetrahydroborate; methanesulfonic acid / diethylene glycol dimethyl ether; isopropyl alcohol / 12 h / 0 - 5 °C 2.2: 0.5 h / 0 - 5 °C 2.3: 3 h / 15 °C View Scheme |
-
-
6921-34-2
benzylmagnesium chloride

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: tetrahydrofuran / 0 - 20 °C 2.1: sodium tetrahydroborate; methanesulfonic acid / tetrahydrofuran; isopropyl alcohol / 0 - 10 °C 2.2: 4.75 h / 0 - 15 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: 10 h / 5 °C 2.1: sodium tetrahydroborate; methanesulfonic acid / 1.5 h / 6 °C 2.2: 1.5 h / 6 °C View Scheme |

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 10 h / 5 °C 2.1: sodium tetrahydroborate; methanesulfonic acid / 1.5 h / 6 °C 2.2: 1.5 h / 6 °C View Scheme |
-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 0 - 20℃; for 24h; | 100% |
-
-
24601-74-9
(S)-4-isopropyloxazolidine-2,5-dione

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at -15℃; for 4h; Inert atmosphere; | 97% |
-
-
6949-76-4
1-((methoxycarbonyl)amino)cyclopentane-1-carboxylic acid

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 0 - 20℃; for 4h; | 95% |
-
-
13335-71-2
2-(2,6-dimethylphenoxy)acetic acid

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

-
-
898254-22-3
N-((2S,4S,5S)-5-(dibenzylamino)-4-hydroxy-1,6-diphenylhexan-2-yl)-2-(2,6-dimethylphenoxy)acetamide

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 0.5h; | 90% |
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 4h; | 86% |
-
-
1239660-25-3
(S)-2-cyclopentyl-2-((methoxycarbonyl)amino)acetic acid

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 0 - 20℃; for 4h; | 90% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

-
-
162849-93-6
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-(t-butyloxycarbonylamino)-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| With potassium carbonate at 16℃; for 11h; | 88% |
-
-
162537-11-3
(S)-2-(methoxycarbonylamino)-3,3-dimethylbutanoic acid

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 0 - 20℃; for 4h; | 87% |
| Stage #1: (S)-2-(methoxycarbonylamino)-3,3-dimethylbutanoic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane; N,N-dimethyl-formamide at 0℃; for 0.25h; Stage #2: (2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane With N-ethyl-N,N-diisopropylamine In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 24h; Further stages.; |
-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

-
-
74-88-4
methyl iodide

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 20℃; for 5h; | 17% |
-
-
1066876-06-9
2-oxo-3-phenyl-oxazolidine-5-carbonyl chloride

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 8h; |
-
-
1066875-99-7
2-oxo-3-phenyl-oxazolidine-5-carbonyl chloride

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 8h; |
-
-
1066876-02-5
2-oxo-3-(3-trifluoromethyl-phenyl)-oxazolidine-5-carbonyl chloride

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 8h; |
-
-
96-81-1
N-acetyl-L-valine

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane; water at 0 - 4℃; for 24h; |
-
-
192800-77-4
2S-(1-tetrahydro-pyrimid-2-onyl)-3-methyl butanoyl chloride

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

-
-
192726-04-8
(2S,3S,5S)-2-N,N-dibenzylamino-3-hydroxy-5-(2S-(2-oxotetrahydropyrimidin-2-yl)-3-methylbutanoyl)amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| With 1H-imidazole In ethyl acetate; N,N-dimethyl-formamide at 0 - 20℃; | |
| With 1H-imidazole In DMF (N,N-dimethyl-formamide); ethyl acetate at 2℃; | |
| With 1H-imidazole In ethyl acetate at 1℃; for 1.5h; |
-
-
20143-48-0
2,6-dimethylphenoxyacetyl chloride

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

-
-
898254-22-3
N-((2S,4S,5S)-5-(dibenzylamino)-4-hydroxy-1,6-diphenylhexan-2-yl)-2-(2,6-dimethylphenoxy)acetamide

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In ethyl acetate at 20℃; for 1h; |
-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

-
-
192726-05-9
(2S,3S,5S)-2-amino-3-hydroxy-5-(2S-(2-oxotetrahydropyrimidin-2-yl)-3-methylbutanoyl)amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: imidazole / dimethylformamide; ethyl acetate / 0 - 20 °C 2: HCO2NH4 / Pd/C / methanol / 50 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: 1,1'-carbonyldiimidazole / ethyl acetate / 2 h / 15 - 20 °C 1.2: 4 h / 70 - 75 °C 2.1: 5%-palladium/activated carbon; ammonium formate / methanol / 2 h / 50 - 55 °C View Scheme |
-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: EDCl; HOBt / H2O; CH2Cl2 / 24 h / 0 - 4 °C 2: HCO2NH4 / Pd/C / methanol / 50 °C View Scheme |
-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

-
-
854754-08-8
methyl (1S)-1-({[(1S,3S,4S)-4-amino-1-benzyl-3-hydroxy-5-phenylpentyl]amino}carbonyl)-2,2-dimethylpropylcarbamate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: EDCl; HOBt / dimethylformamide; CH2Cl2 / 0.25 h / 0 °C 1.2: DIPEA / dimethylformamide; CH2Cl2 / 24 h / 0 - 20 °C 2.1: HCO2NH4 / Pd/C / methanol / 50 °C View Scheme | |
| Multi-step reaction with 2 steps 1: N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate / N,N-dimethyl-formamide / 4 h / 0 - 20 °C 2: ammonium formate; palladium 10% on activated carbon / methanol / 15 h / 50 °C View Scheme |
-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: EDCl; HOBt / H2O; CH2Cl2 / 24 h / 0 - 4 °C 2: HCO2NH4 / Pd/C / methanol / 50 °C 3: Et3N / tetrahydrofuran / 8 h / 0 - 20 °C View Scheme |
-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: EDCl; HOBt / H2O; CH2Cl2 / 24 h / 0 - 4 °C 2: HCO2NH4 / Pd/C / methanol / 50 °C 3: Et3N / tetrahydrofuran / 8 h / 0 - 20 °C View Scheme |
-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: EDCl; HOBt / H2O; CH2Cl2 / 24 h / 0 - 4 °C 2: HCO2NH4 / Pd/C / methanol / 50 °C 3: Et3N / tetrahydrofuran / 8 h / 0 - 20 °C View Scheme |
-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: EDCl; HOBt / H2O; CH2Cl2 / 24 h / 0 - 4 °C 2: HCO2NH4 / Pd/C / methanol / 50 °C 3: Et3N / tetrahydrofuran / 8 h / 0 - 20 °C View Scheme |
-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: EDCl; HOBt / H2O; CH2Cl2 / 24 h / 0 - 4 °C 2: HCO2NH4 / Pd/C / methanol / 50 °C 3: Et3N / tetrahydrofuran / 8 h / 0 - 20 °C View Scheme |
-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: imidazole / dimethylformamide; ethyl acetate / 0 - 20 °C 2: HCO2NH4 / Pd/C / methanol / 50 °C 3: Et3N / tetrahydrofuran / 8 h / 0 - 20 °C View Scheme |
-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: EDCl; HOBt / dimethylformamide; CH2Cl2 / 0.25 h / 0 °C 1.2: DIPEA / dimethylformamide; CH2Cl2 / 24 h / 0 - 20 °C 2.1: HCO2NH4 / Pd/C / methanol / 50 °C 3.1: Et3N / tetrahydrofuran / 8 h / 0 - 20 °C View Scheme |
-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

-
-
144163-85-9
(2S,3S,5S)-2-amino-3-hydroxy-5-(t-butyloxycarbonylamino)-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Pd(OH)2; HCO2NH4 View Scheme | |
| Multi-step reaction with 2 steps 1: tetrahydrofuran / 1 h / 20 °C 2: palladium on activated charcoal; ammonium formate; water / ethanol / 3 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: tetrahydrofuran / 1 h / 20 °C 2: palladium 10% on activated carbon; ammonium formate / ethanol; water / 3 h / Reflux View Scheme |
-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

-
-
192725-49-8
(2S,3S,5S)-2-(2,6-dimethylphenoxyacetyl)amino-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: Pd(OH)2; HCO2NH4 2: EDC 3: trifluoroacetic acid View Scheme | |
| Multi-step reaction with 4 steps 1: tetrahydrofuran / 1 h / 20 °C 2: palladium on activated charcoal; ammonium formate; water / ethanol / 3 h / Reflux 3: triethylamine; O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate / N,N-dimethyl-formamide / 10 h / 20 °C 4: trifluoroacetic acid / dichloromethane / 0.5 h View Scheme |
-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

-
-
192725-45-4
(2S,3S,5S)-2-(2,6-dimethylphenoxyacetyl)amino-3-hydroxy-5-(t-butyloxycarbonylamino)-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: Pd(OH)2; HCO2NH4 2: EDC View Scheme | |
| Multi-step reaction with 3 steps 1: tetrahydrofuran / 1 h / 20 °C 2: palladium on activated charcoal; ammonium formate; water / ethanol / 3 h / Reflux 3: triethylamine; O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate / N,N-dimethyl-formamide / 10 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: tetrahydrofuran / 1 h / 20 °C 2: palladium 10% on activated carbon; ammonium formate / ethanol; water / 3 h / Reflux 3: 2-(3H-[1,2,3]triazolo[4,5-b]pyridin-3-yl)-1,1,3,3-tetramethylisouronium hexafluorophosphate; triethylamine / N,N-dimethyl-formamide / 10 h / 20 °C View Scheme |
(2S,3S,5S)-5-Amino-2-(benzylamino)-1,6-diphenylhexan-3-ol Chemical Properties
Molecule structure of (2S,3S,5S)-5-Amino-2-(benzylamino)-1,6-diphenylhexan-3-ol (CAS NO.156732-15-9):
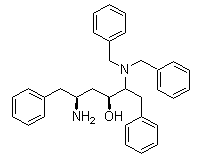
Molecular Weight: 464.64 g/mol
Molecular Formula: C32H36N2O
Density: 1.125 g/cm3
Boiling Point: 642.878 °C at 760 mmHg
Flash Point: 342.601 °C
Index of Refraction: 1.624
Molar Refractivity: 145.729 cm3
Molar Volume: 412.963 cm3
Polarizability: 57.772×10-24 cm3
Surface Tension: 50.823 dyne/cm
Enthalpy of Vaporization: 99.705 kJ/mol
InChI: InChI=1/C32H36N2O/c33-30(21-26-13-5-1-6-14-26)23-32(35)31(22-27-15-7-2-8-16-27)34(24-28-17-9-3-10-18-28)25-29-19-11-4-12-20-29/h1-20,30-32,35H,21-25,33H2/t30-,31-,32-/m0/s1
InChIKey: SRVJQTROLWUZRB-CPCREDONBR
Product Categories: Aromatics Compounds;Aromatics;Chiral Reagents;Intermediates
(2S,3S,5S)-5-Amino-2-(benzylamino)-1,6-diphenylhexan-3-ol Uses
(2S,3S,5S)-5-Amino-2-(benzylamino)-1,6-diphenylhexan-3-ol (CAS NO.156732-15-9) is used as an intermediate in the synthesis of Ritonavir.
(2S,3S,5S)-5-Amino-2-(benzylamino)-1,6-diphenylhexan-3-ol Specification
(2S,3S,5S)-5-Amino-2-(benzylamino)-1,6-diphenylhexan-3-ol (CAS NO.156732-15-9) is also named as (2S,3S,5S)-5-amino-2-(dibenzylamino)-3-hydroxy-1,6-diphenylhexane ; Benzenebutanol,g-amino-a-[1-[bis(phenylmethyl)amino]-2-phenylethyl]-,[aS-[aR*(R*),gR*]]- .
Related Products
- (2S)-(+)-2,5-Dihydro-3,6-dimethoxy-2-isopropylpyrazine
- (2S)-(+)-Glycidyl tosylate
- (2S)-(1-Tetrahydropyramid-2-one)-3-methylbutanoic acid
- (2S)-(3R,5S)-Adamantan-1-yl{[(1R)-2-hydroxy-1-phenylethyl]amino}acetonitrile
- (2S)-{[(Benzyloxy)carbonyl]amino}(cyclohexyl)acetic acid
- (2S)-1-(1,2-Dioxo-3,3-dimethylpentyl)-2-pyrrolidinecarboxylic acid
- (2S)-1-(3-Acetylthio-2-methyl-1-oxopropyl)-L-proline
- (2S)-1-(Chloroacetyl)-2-pyrrolidinecarbonitrile
- (2S)-1,4-Dioxan-2-yl-methanol
- (2S)-1-[(9H-Fluoren-9-ylmethoxy)carbonyl]piperidine-2-carboxylic acid
- 156733-09-4
- 15673-79-7
- 1567-38-0
- 156740-57-7
- 156-74-1
- 15674-58-5
- 15674-67-6
- 156755-23-6
- 156755-25-8
- 156755-27-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View