-
Name
(1H-INDOL-3-YL)-OXO-ACETIC ACID ETHYL ESTER
- EINECS 256-954-5
- CAS No. 51079-10-8
- Article Data32
- CAS DataBase
- Density 1.278 g/cm3
- Solubility
- Melting Point
- Formula C12H11NO3
- Boiling Point 391.7 °C at 760 mmHg
- Molecular Weight 217.224
- Flash Point 190.7 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Ethyl 1H-indol-3-yl(oxo)acetate;Ethyl 2-(1H-indol-3-yl)-2-oxoacetate;Ethylindole-3-glyoxylate;
- PSA 59.16000
- LogP 1.91370
1H-Indole-3-aceticacid, α-oxo-, ethyl ester Specification
The 1H-Indole-3-aceticacid, α-oxo-, ethyl ester, with the CAS registry number 51079-10-8, is also known as Ethyl indole-3-glyoxylate. Its EINECS number is 256-954-5. This chemical's molecular formula is C12H11NO3 and molecular weight is 217.22. What's more, its IUPAC name is ethyl 2-(1H-indol-3-yl)-2-oxoacetate.
Physical properties of 1H-Indole-3-aceticacid, α-oxo-, ethyl ester are: (1)ACD/LogP: 1.73; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.72; (4)ACD/LogD (pH 7.4): 1.72; (5)ACD/BCF (pH 5.5): 12.04; (6)ACD/BCF (pH 7.4): 12.04; (7)ACD/KOC (pH 5.5): 206.64; (8)ACD/KOC (pH 7.4): 206.64; (9)#H bond acceptors: 4 #H ; (10)bond donors: 1; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 48.3 Å2; (13)Index of Refraction: 1.618; (14)Molar Refractivity: 59.59 cm3; (15)Molar Volume: 169.8 cm3; (16)Polarizability: 23.62×10-24cm3; (17)Surface Tension: 53.8 dyne/cm; (18)Density: 1.278 g/cm3; (19)Flash Point: 190.7 °C; (20)Enthalpy of Vaporization: 64.14 kJ/mol; (21)Boiling Point: 391.7 °C at 760 mmHg; (22)Vapour Pressure: 2.41E-06 mmHg at 25°C.
Preparation: this chemical can be prepared by 3-ethoxycarbonecarbonyl-indole-1-carboxylic acid tert-butyl ester at the temperature of 180-185°C. This reaction will need solvent neat (no solvent) with the reaction time of 30 min. The yield is about 99%.
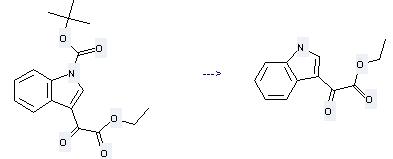
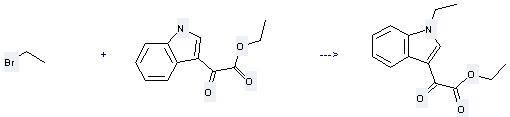
Uses of 1H-Indole-3-aceticacid, α-oxo-, ethyl ester: it can be used to produce ethyl 1-ethylindole-3-glyoxalate at the ambient temperature. It will need reagents NaH, tris<2-(2-methoxyethoxy)ethyl>amine and solvent CH2Cl2 with the reaction time of 24 hours. The yield is about 62%.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OCC)C(=O)c2c1ccccc1nc2
(2)Std. InChI: InChI=1S/C12H11NO3/c1-2-16-12(15)11(14)9-7-13-10-6-4-3-5-8(9)10/h3-7,13H,2H2,1H3
(3)Std. InChIKey: WTPMFFQBDYIARF-UHFFFAOYSA-
Related Products
- 1H-Indole-3-aceticacid, 2,3-dihydro-2-oxo-
- 1H-Indole-3-aceticacid, 2,7-dimethyl-
- 1H-Indole-3-aceticacid, 2-methyl-, ethyl ester
- 1H-Indole-3-aceticacid, 5-bromo-a-oxo-, ethyl ester
- 1H-Indole-3-aceticacid, 5-chloro-2-methyl-
- 1H-Indole-3-aceticacid, 5-cyano-2-methyl-
- 1H-Indole-3-aceticacid, 5-ethoxy-2-methyl-
- 1H-Indole-3-aceticacid, 5-ethyl-
- 1H-Indole-3-aceticacid, 5-methyl-, hydrazide
- 1H-Indole-3-aceticacid, hydrazide
- 51084-83-4
- 5108-69-0
- 5108-96-3
- 51094-17-8
- 51094-45-2
- 51094-78-1
- 51096-49-2
- 510-99-6
- 5109-96-6
- 5109-97-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View