-
Name
2'-Methoxycinnamaldehyde
- EINECS 216-131-3
- CAS No. 1504-74-1
- Article Data17
- CAS DataBase
- Density 1.068 g/cm3
- Solubility Soluble in alcohol, ether, benzene, insoluble in water
- Melting Point 44-48 °C(lit.)
- Formula C10H10O2
- Boiling Point 334.8 °C at 760 mmHg
- Molecular Weight 162.188
- Flash Point 134.4 °C
- Transport Information
- Appearance Colorless crystal
- Safety 26-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Cinnamaldehyde,o-methoxy- (6CI,7CI,8CI);3-(2-Methoxyphenyl)-2-propenal;3-(2-Methoxyphenyl)acrylaldehyde;NSC 114599;o-Methoxycinnamaldehyde;o-Methoxycinnamic aldehyde;
- PSA 26.30000
- LogP 1.90730
2'-Methoxycinnamaldehyde Consensus Reports
Reported in EPA TSCA Inventory.
2'-Methoxycinnamaldehyde Specification
The o-Methoxy cinnamaldehyde with CAS registry number of 1504-74-1 is also known as 3-(2-Methoxyphenyl)acrylaldehyde. The IUPAC name is 3-(2-Methoxyphenyl)prop-2-enal. Its EINECS registry number is 216-131-3. In addition, the formula is C10H10O2 and the molecular weight is 162.19. This chemical is a colorless crystal and should be sealed in cool and dry place. What's more, it is used as organic synthesis intermediate, and is also used for the production of spices, medicine.
Physical properties about o-Methoxy cinnamaldehyde are: (1)ACD/LogP: 2.13; (2)ACD/LogD (pH 5.5): 2.13; (3)ACD/LogD (pH 7.4): 2.13; (4)ACD/BCF (pH 5.5): 24.42; (5)ACD/BCF (pH 7.4): 24.42; (6)ACD/KOC (pH 5.5): 342.75; (7)ACD/KOC (pH 7.4): 342.75; (8)#H bond acceptors: 2; (9)#Freely Rotating Bonds: 3; (10)Index of Refraction: 1.559; (11)Molar Refractivity: 49 cm3; (12)Molar Volume: 151.7 cm3; (13)Surface Tension: 37.6 dyne/cm; (14)Density: 1.068 g/cm3; (15)Flash Point: 134.4 °C; (16)Enthalpy of Vaporization: 57.78 kJ/mol; (17)Boiling Point: 334.8 °C at 760 mmHg; (18)Vapour Pressure: 0.000125 mmHg at 25 °C.
Preparation of o-Methoxy cinnamaldehyde: it is prepared by methylation reaction of salicylaldehyde with dimethyl sulfate. Firstly, 30% sodium hydroxide solution is added to salicylaldehyde solution under stirring. The mixture is heated to boiling. Secondly, dimethyl sulfate is slowly added for 3 hours and the mixture is refluxed for another 2 or 3 hours. Then oil layer is cooled, washed with 5% sodium hydroxide solution and washed with water to pH 8. At last, product is obtained by drying and vacuum distillation. The yield is about 60%.
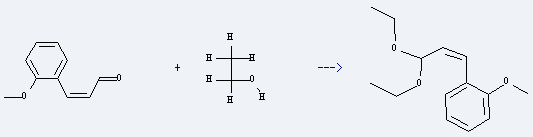
Uses of o-Methoxy cinnamaldehyde: it is used to produce 1-(3,3-diethoxy-propenyl)-2-methoxy-benzene by reaction with ethanol. The reaction occurs with catalyst I2 for 1 hour. The yield is about 98%.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: COC1=CC=CC=C1C=CC=O
2. InChI: InChI=1S/C10H10O2/c1-12-10-7-3-2-5-9(10)6-4-8-11/h2-8H,1H3
3. InChIKey: KKVZAVRSVHUSPL-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | > 2gm/kg (2000mg/kg) | Japanese Kokai Tokyo Koho Patents. Vol. #92-360829, | |
| rabbit | LD50 | skin | > 5gm/kg (5000mg/kg) | Food and Cosmetics Toxicology. Vol. 13, Pg. 845, 1975. | |
| rat | LD50 | oral | > 5gm/kg (5000mg/kg) | Food and Cosmetics Toxicology. Vol. 13, Pg. 845, 1975. |
Related Products
- 2-Methoxycinnamaldehyde
- 150-50-5
- 150516-43-1
- 150517-75-2
- 150517-76-3
- 150517-77-4
- 15051-81-7
- 150520-10-8
- 150521-32-7
- 150522-09-1
- 15052-28-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View