-
Name
3,5-DI-T-BUTYL-4-METHOXYBENZALDEHYDE
- EINECS
- CAS No. 5221-17-0
- Article Data24
- CAS DataBase
- Density 2.114g/cm3
- Solubility
- Melting Point
- Formula C3H4 Br2 O
- Boiling Point 191.9°Cat760mmHg
- Molecular Weight 215.872
- Flash Point 88°C
- Transport Information
- Appearance
- Safety Poison by intravenous route. Mutagenic data reported. When heated to decomposition it emits toxic fumes of Br−. See also ALDEHYDES and BROMIDES.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Propionaldehyde,2,3-dibromo- (7CI,8CI); 2,3-Dibromopropanal; 2,3-Dibromopropional;2,3-Dibromopropionaldehyde; Acrolein dibromide; NSC 6737
- PSA 17.07000
- LogP 1.34370
Synthetic route

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane at 0℃; Cooling with ice; | 80% |
| With bromine Ambient temperature; competitive bromination with 1-heptene; other unsaturated carbonyl compound; | |
| With bromine |
-
-
18791-02-1
2,3-dibromo-propionyl chloride

-
-
5221-17-0
2,3-dibromopropanal

| Conditions | Yield |
|---|---|
| With lithium tri(t-butoxy)aluminum hydride In tetrahydrofuran Reduction; | 79% |

| Conditions | Yield |
|---|---|
| bei der Ozonspaltung; |

| Conditions | Yield |
|---|---|
| With water; bromine |

| Conditions | Yield |
|---|---|
| With bromine |
-
-
85858-56-6
4,5-dibromo-pent-2-enoic acid

-
-
67-66-3
chloroform

-
-
7732-18-5
water

-
-
10028-15-6
ozone

-
A
-
5221-17-0
2,3-dibromopropanal

-
-
5221-17-0
2,3-dibromopropanal

-
-
35933-97-2
N-(1-trifluoromethyltrifluoroethylidene)-2-trifluoromethyl-3,3,3-trifluoropropionamide

-
-
83926-93-6
2-(1,2-dibromoethyl)-4,4-bis(trifluoromethyl)-6-(2-hydrohexafluoroisopropyl)-2H,4H-1,3,5-dioxazine

| Conditions | Yield |
|---|---|
| In tetrachloromethane at 100℃; for 1h; | 94.8% |
-
-
5221-17-0
2,3-dibromopropanal

-
-
17660-57-0
meso-2,3-Dimercaptobernsteinsaeure-dimethylester

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid | 90% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; for 20h; | 76% |
-
-
5221-17-0
2,3-dibromopropanal

-
-
504-63-2
trimethyleneglycol

-
-
89791-57-1
2-(1,2-dibromoethyl)-1,3-dioxane

| Conditions | Yield |
|---|---|
| With cationite KU-2-8 In benzene Heating; | 71% |
| With potassium carbonate; toluene-4-sulfonic acid 1.0 CH2Cl2, reflux, 5 h, 2.) heating.; Multistep reaction; |
-
-
5221-17-0
2,3-dibromopropanal

-
-
107-21-1
ethylene glycol

-
-
5267-72-1
2-(1',2'-dibromoethyl)-1,3-dioxolane

| Conditions | Yield |
|---|---|
| With cationite KU-2-8 In benzene Heating; | 71% |

| Conditions | Yield |
|---|---|
| With cationite KU-2-8 In benzene Heating; | 64% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
60-29-7
diethyl ether

-
-
5221-17-0
2,3-dibromopropanal

-
-
25109-57-3
3,4-dibromobutan-2-one

-
-
186581-53-3, 908094-01-9
diazomethane

-
-
5221-17-0
2,3-dibromopropanal

-
-
25109-57-3
3,4-dibromobutan-2-one

| Conditions | Yield |
|---|---|
| With diethyl ether |



| Conditions | Yield |
|---|---|
| und Behandeln des erhaltenen Reaktionsprodukts mit 2,5,6-Triamino-3H-pyrimidin-4-on und KI; |
-
-
67-56-1
methanol

-
-
5221-17-0
2,3-dibromopropanal

-
-
15755-09-6
formimidomethylester hydrochloride

-
-
248955-54-6
1,2-dibromo-3,3-dimethoxypropane

-
-
1073-99-0
4,5,6-triamino-1H-pyrimidine-2-thione

-
-
5221-17-0
2,3-dibromopropanal

-
-
4271-30-1
N-(4-aminobenzoyl)-L-glutamic acid

-
-
103207-69-8
N-{4-[(4-amino-2-thioxo-1,2-dihydro-pteridin-6-ylmethyl)-amino]-benzoyl}-L-glutamic acid

-
-
1073-99-0
4,5,6-triamino-1H-pyrimidine-2-thione

-
-
5221-17-0
2,3-dibromopropanal

-
-
150-13-0
4-amino-benzoic acid

-
-
92253-82-2
4-[(4-amino-2-thioxo-1,2-dihydro-pteridin-6-ylmethyl)-amino]-benzoic acid

-
-
3240-72-0
5,6-diaminouracil

-
-
5221-17-0
2,3-dibromopropanal

-
-
4271-30-1
N-(4-aminobenzoyl)-L-glutamic acid

-
-
25663-25-6
N-{4-[(2,4-dioxo-1,2,3,4-tetrahydro-pteridin-6-ylmethyl)-amino]-benzoyl}-L-glutamic acid

| Conditions | Yield |
|---|---|
| With sodium acetate; acetic acid |

-
-
7377-08-4
N-(4-aminobenzoyl)-B-alanine

-
-
1004-75-7
2,5,6-triamino-3,4-dihydro-4-pyrimidinone

-
-
5221-17-0
2,3-dibromopropanal

-
-
15677-93-7
N-pteroyl-β-alanine

| Conditions | Yield |
|---|---|
| With sodium dichromate | |
| With iodine |

-
-
1004-75-7
2,5,6-triamino-3,4-dihydro-4-pyrimidinone

-
-
5221-17-0
2,3-dibromopropanal

-
-
10541-83-0
N-methyl-p-aminobenzoic acid

-
-
5623-18-7
10-methyl-pteroic acid

-
-
1004-75-7
2,5,6-triamino-3,4-dihydro-4-pyrimidinone

-
-
5221-17-0
2,3-dibromopropanal

-
-
4740-24-3
4-(N-butylamino)benzoic acid

-
-
1004-75-7
2,5,6-triamino-3,4-dihydro-4-pyrimidinone

-
-
5221-17-0
2,3-dibromopropanal

-
-
99146-89-1
N-(4-amino-benzoyl)-alanine

| Conditions | Yield |
|---|---|
| With sodium dichromate | |
| With iodine |

-
-
1004-75-7
2,5,6-triamino-3,4-dihydro-4-pyrimidinone

-
-
5221-17-0
2,3-dibromopropanal

-
-
52980-68-4
p-(N-methylamino)benzoyl-L-glutamic acid

-
-
2410-93-7
N10-methylfolic acid

-
-
1004-75-7
2,5,6-triamino-3,4-dihydro-4-pyrimidinone

-
-
5221-17-0
2,3-dibromopropanal

-
-
875430-61-8
N-(4-amino-3-methyl-benzoyl)-L-glutamic acid

-
-
4230-33-5
N-(4-aminobenzoyl)-glutamic acid

-
-
1004-75-7
2,5,6-triamino-3,4-dihydro-4-pyrimidinone

-
-
5221-17-0
2,3-dibromopropanal

-
-
65165-92-6
folate


| Conditions | Yield |
|---|---|
| With iodine | |
| With sodium dichromate |

-
-
1004-75-7
2,5,6-triamino-3,4-dihydro-4-pyrimidinone

-
-
5221-17-0
2,3-dibromopropanal

-
-
150-13-0
4-amino-benzoic acid

-
-
119-24-4
pteroic acid

| Conditions | Yield |
|---|---|
| With ethanol Loesung vom pH 4; |

-
-
1004-75-7
2,5,6-triamino-3,4-dihydro-4-pyrimidinone

-
-
5221-17-0
2,3-dibromopropanal

-
-
95-53-4
o-toluidine

| Conditions | Yield |
|---|---|
| With sodium dichromate |

-
-
98-50-0
p-aminophenylarsonic acid

-
-
1004-74-6
2,4,5,6-tetraaminopyrimidine

-
-
5221-17-0
2,3-dibromopropanal

-
-
109160-53-4
{4-[(2,4-diamino-pteridin-6-ylmethyl)-amino]-phenyl}-arsonic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium dichromate; acetic acid pH 3-4; |

-
-
1004-74-6
2,4,5,6-tetraaminopyrimidine

-
-
5221-17-0
2,3-dibromopropanal

-
-
879278-42-9
N-(4-amino-benzoyl)-serine

| Conditions | Yield |
|---|---|
| With water; iodine |

-
-
4230-33-5
N-(4-aminobenzoyl)-glutamic acid

-
-
1431-40-9
2-methylsulfanyl-pyrimidine-4,5,6-triamine

-
-
5221-17-0
2,3-dibromopropanal

-
-
94543-82-5
N-{4-[(4-amino-2-methylsulfanyl-pteridin-6-ylmethyl)-amino]-benzoyl}-DL-glutamic acid

| Conditions | Yield |
|---|---|
| With sodium acetate |

2,3-Dibromopropanal Chemical Properties
IUPAC Name: 2,3-Dibromopropanal
Product Name: 2,3-Dibromopropanal
The MF of 2,3-Dibromopropanal (CAS NO.5221-17-0) is C3H4Br2O.
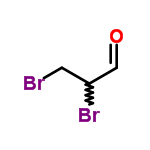
The MW of 2,3-Dibromopropanal (CAS NO.5221-17-0) is 215.8713.
Synonyms of 2,3-Dibromopropanal (CAS NO.5221-17-0): Propanal, 2,3-dibromo- ; 2,3-Dibromopropionaldehyde ; Propanal, 2,3-dibromo- (9CI) ; Propionaldehyde, 2,3-dibromo-
Product Categories: Pharmaceutical Intermediates
Form: Light yellow fuming liquid
EINECS: 226-017-5
Index of Refraction: 1.53
Density: 2.114 g/ml
Flash Point: 88 °C
Boiling Point: 191.9 °C
2,3-Dibromopropanal Uses
2,3-Dibromopropanal (CAS NO.5221-17-0) is anti-tumor class medicine for medical methylamine neopterin production,also for other organic synthesis intermediates.
2,3-Dibromopropanal Production
Preparation Products: Folic acid
Raw materials: Carbon tetrachloride-->Acrolein
2,3-Dibromopropanal Toxicity Data With Reference
| 1. | mmo-sat 1 nmol/plate | MUREAV Mutation Research. 78 (1980),113. | ||
| 2. | ipr-mus LD50:5 mg/kg | JAFCAU Journal of Agricultural and Food Chemistry. 30 (1982),627. | ||
| 3. | ivn-mus LD50:56 mg/kg | CSLNX* U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. (Aberdeen Proving Ground, MD 21010) NX#02408 . |
2,3-Dibromopropanal Safety Profile
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 52212-02-9
- 522-12-3
- 52213-27-1
- 5221-42-1
- 52214-84-3
- 5221-49-8
- 52215-41-5
- 5221-62-5
- 52217-52-4
- 52217-53-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View