-
Name
2,4'-DICHLOROBIPHENYL
- EINECS
- CAS No. 34883-43-7
- Article Data22
- CAS DataBase
- Density 1.249g/cm3
- Solubility 0.62mg/L(25 oC)
- Melting Point 46°C
- Formula C12H8 Cl2
- Boiling Point 303.3°Cat760mmHg
- Molecular Weight 223.102
- Flash Point 133.6°C
- Transport Information
- Appearance
- Safety Low toxicity by ingestion. When heated to decomposition it emits toxic vapors of Cl−.
- Risk Codes 33-50/53
-
Molecular Structure
-
Hazard Symbols

- Synonyms Biphenyl,2,4'-dichloro- (7CI); 2,4'-Dichlorobiphenyl; CB 8; PCB 8
- PSA 0.00000
- LogP 4.66040
Synthetic route
-
-
92-52-4
biphenyl

-
A
-
2051-60-7
2-chloro-1,1'-biphenyl

-
B
-
34883-43-7
2,4'-dichlorobiphenyl

-
C
-
2050-68-2
4,4'-dichlorobiphenyl

-
D
-
2051-62-9
4'-biphenyl chloride

| Conditions | Yield |
|---|---|
| With chlorine; K-Zeolith L In dichloromethane at 20℃; for 6h; Product distribution; further zeolithes; further solvents and reaction conditions; | A 1.9% B 4.9% C 89% D 3% |
| With chlorine; K-Zeolith L In dichloromethane at 20℃; for 6h; | A 1.9% B 4.9% C 89% D 3% |
| With chlorine; K-Zeolith L In tetrachloromethane at 20℃; for 6h; Title compound not separated from byproducts; | A 10% B 14.7% C 66.5% D 5.7% |
| With chlorine; K-Zeolith L In various solvent(s) at 20℃; for 6h; Title compound not separated from byproducts; | A 10.1% B 5.4% C 39.7% D 35.9% |
| With chlorine; K-Zeolith L In acetic acid at 40℃; for 6h; Title compound not separated from byproducts; | A 28.9% B 8.8% C 13% D 31.9% |


| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); caesium carbonate; 1,3,5,7-tetramethyl-8-phenyl-2,4,6-trioxa-8-phosphatricyclo[3.3.1.13,7]decane In toluene at 70℃; Suzuki coupling; Inert atmosphere; chemoselective reaction; | 64% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-Bromo-2-iodobenzene With n-butyllithium In tetrahydrofuran; hexane at -70℃; Flow reactor; Stage #2: 4-chlorophenyl lithium In tetrahydrofuran at 0℃; Flow reactor; Stage #3: With hexachloroethane In tetrahydrofuran; hexane at -30℃; Flow reactor; | 64% |
-
-
108-90-7
chlorobenzene

-
-
108-95-2
phenol

-
A
-
25569-80-6
2,3'-dichloro-1,1'-biphenyl

-
B
-
34883-43-7
2,4'-dichlorobiphenyl

-
C
-
2974-90-5
3,4'-dichlorobiphenyl

-
D
-
2050-68-2
4,4'-dichlorobiphenyl

-
E
-
2050-67-1
PCB 11

-
F
-
13029-08-8
2,2'-Dichlorobiphenyl

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; iodine at 289.9℃; for 0.0272222h; Product distribution; | A 34.4% B 18.7% C 15.5% D 3.9% E 13.1% F 14.4% |

-
-
492-17-1
2,4'-diaminobiphenyl

-
-
34883-43-7
2,4'-dichlorobiphenyl

| Conditions | Yield |
|---|---|
| With sulfuric acid Diazotization.Behandlung der Diazoniumsalz-Loesung mit Hg(NO3)2 und KCl und Erhitzen des erhaltenen Diazonium-tetrachloromercurats(II) mit KCl bis auf 120grad; |
-
-
138588-57-5
3-chloro-4-(4-chlorophenyl)benzenamine

-
-
34883-43-7
2,4'-dichlorobiphenyl

| Conditions | Yield |
|---|---|
| With sulfuric acid Diazotization.Behandlung der Diazoniumsalz-Loesung mit H3PO2; |
-
-
673-41-6
p-chlorobenzenediazonium tetrafluoroborate

-
A
-
34883-43-7
2,4'-dichlorobiphenyl

-
B
-
2974-90-5
3,4'-dichlorobiphenyl

-
C
-
141320-17-4
(4-chloro-phenyl)-(5,4'-dichloro-biphenyl-2-yl)-diazene

-
D
-
108-90-7
chlorobenzene

-
E
-
1602-00-2
bis-(4-chloro-phenyl)-diazene

| Conditions | Yield |
|---|---|
| With titanium(III) sulphate In water; acetonitrile at 0 - 5℃; Mechanism; Product distribution; other solvents, p-chlorobenzenediazonium sulfate and benzenediazonium salts; | A 0.7 % Chromat. B 6.5 % Chromat. C 63.5 % Chromat. D 5.8 % Chromat. E 5.3 % Chromat. |

-
-
106-47-8
4-chloro-aniline

-
-
108-90-7
chlorobenzene

-
A
-
34883-43-7
2,4'-dichlorobiphenyl

-
B
-
2974-90-5
3,4'-dichlorobiphenyl

-
C
-
2050-68-2
4,4'-dichlorobiphenyl

| Conditions | Yield |
|---|---|
| With sodium nitrite; trichloroacetic acid; copper(l) chloride at 85℃; for 2h; Yield given. Yields of byproduct given; |

-
-
10312-71-7
1,6-bis-(4-chlorophenyl)-3,4-diacetyl-1,5-hexazadiene

-
-
108-90-7
chlorobenzene

-
A
-
3148-73-0
Diacetylhydrazin

-
B
-
34883-43-7
2,4'-dichlorobiphenyl

-
C
-
2974-90-5
3,4'-dichlorobiphenyl

-
D
-
2050-68-2
4,4'-dichlorobiphenyl

-
E
-
82408-82-0
1-acetyl-1-p-chlorophenylhydrazine

| Conditions | Yield |
|---|---|
| at 131℃; Product distribution; Mechanism; thermolysis, var.: photolysis; | |
| at 10℃; for 6h; Product distribution; Mechanism; Irradiation; |

-
-
673-41-6
p-chlorobenzenediazonium tetrafluoroborate

-
-
108-90-7
chlorobenzene

-
A
-
34883-43-7
2,4'-dichlorobiphenyl

-
B
-
2974-90-5
3,4'-dichlorobiphenyl

-
C
-
2050-68-2
4,4'-dichlorobiphenyl

| Conditions | Yield |
|---|---|
| With potassium acetate; 18-crown-6 ether for 1.5h; Ambient temperature; Yield given. Yields of byproduct given; | |
| With potassium acetate; 18-crown-6 ether for 1.5h; Product distribution; Ambient temperature; other catalysts and reaction conditions; other aromatic reactants; | |
| With pyridine at 23℃; for 18h; Gomberg-Bachman Arylation; Inert atmosphere; Irradiation; Overall yield = 94 percent; Overall yield = 126 mg; |

-
-
79991-27-8
1-p-chlorophenyl-3,4-diacetyl-1-tetrazene

-
-
108-90-7
chlorobenzene

-
A
-
3148-73-0
Diacetylhydrazin

-
B
-
34883-43-7
2,4'-dichlorobiphenyl

-
C
-
2974-90-5
3,4'-dichlorobiphenyl

-
D
-
2050-68-2
4,4'-dichlorobiphenyl

-
E
-
82408-82-0
1-acetyl-1-p-chlorophenylhydrazine

| Conditions | Yield |
|---|---|
| Product distribution; Mechanism; thermolysis, var.: photolysis, 10 degC; | |
| at 10℃; for 2h; Product distribution; Mechanism; Irradiation; |

-
-
10312-71-7
1,6-bis-(4-chlorophenyl)-3,4-diacetyl-1,5-hexazadiene

-
-
108-90-7
chlorobenzene

-
-
67-63-0
isopropyl alcohol

-
A
-
115-10-6
Dimethyl ether

-
B
-
10465-77-7
diacetyl diazene

-
C
-
3148-73-0
Diacetylhydrazin

-
D
-
34883-43-7
2,4'-dichlorobiphenyl

-
E
-
2974-90-5
3,4'-dichlorobiphenyl

-
F
-
2050-68-2
4,4'-dichlorobiphenyl

| Conditions | Yield |
|---|---|
| at 83℃; Product distribution; Mechanism; |

-
-
108-90-7
chlorobenzene

-
A
-
25569-80-6
2,3'-dichloro-1,1'-biphenyl

-
B
-
34883-43-7
2,4'-dichlorobiphenyl

-
C
-
2974-90-5
3,4'-dichlorobiphenyl

-
D
-
2050-68-2
4,4'-dichlorobiphenyl

| Conditions | Yield |
|---|---|
| With thallium(III) trifluoroacetate; palladium diacetate at 135℃; for 50h; Yield given. Further byproducts given. Yields of byproduct given. Title compound not separated from byproducts; | |
| With thallium(III) trifluoroacetate; palladium diacetate at 70℃; for 50h; Yield given. Further byproducts given. Yields of byproduct given. Title compound not separated from byproducts; | |
| With thallium(III) trifluoroacetate; palladium diacetate at 70℃; for 50h; Yield given. Further byproducts given. Yields of byproduct given; |

-
-
108-90-7
chlorobenzene

-
A
-
34883-43-7
2,4'-dichlorobiphenyl

-
B
-
2974-90-5
3,4'-dichlorobiphenyl

-
C
-
2050-68-2
4,4'-dichlorobiphenyl

-
D
-
2050-67-1
PCB 11

| Conditions | Yield |
|---|---|
| With palladium diacetate; thallium(III) trifluoroacetate at 60 - 70℃; Yield given. Further byproducts given. Yields of byproduct given; |

-
-
108-90-7
chlorobenzene

-
A
-
25569-80-6
2,3'-dichloro-1,1'-biphenyl

-
B
-
34883-43-7
2,4'-dichlorobiphenyl

-
C
-
2974-90-5
3,4'-dichlorobiphenyl

-
D
-
2050-68-2
4,4'-dichlorobiphenyl

-
E
-
2050-67-1
PCB 11

-
F
-
13029-08-8
2,2'-Dichlorobiphenyl

| Conditions | Yield |
|---|---|
| With thallium(III) trifluoroacetate; palladium diacetate at 70℃; for 50h; Product distribution; Rate constant; variation of amount of Pd(II) and temperature; | |
| With palladium diacetate; thallium(III) trifluoroacetate at 60 - 70℃; Product distribution; selectivity in the oxidative coupling; |

-
-
108-90-7
chlorobenzene

-
A
-
34883-43-7
2,4'-dichlorobiphenyl

-
B
-
2974-90-5
3,4'-dichlorobiphenyl

-
C
-
2050-68-2
4,4'-dichlorobiphenyl

-
D
-
13029-08-8
2,2'-Dichlorobiphenyl

| Conditions | Yield |
|---|---|
| With thallium(III) trifluoroacetate; palladium diacetate at 135℃; for 50h; Yield given. Further byproducts given. Yields of byproduct given. Title compound not separated from byproducts; |


| Conditions | Yield |
|---|---|
| With copper In N,N-dimethyl-formamide Ulman reaction; Heating; |
-
-
1204-42-8
2′-chlorobiphenyl-4-amine

-
-
34883-43-7
2,4'-dichlorobiphenyl

| Conditions | Yield |
|---|---|
| With copper(I) chloride; cis-nitrous acid Sandmeyer reaction; |

| Conditions | Yield |
|---|---|
| With sodium acetate; cis-nitrous acid Arylation; Gomberg-Bachman reaction; |
-
-
108-90-7
chlorobenzene

-
A
-
25569-80-6
2,3'-dichloro-1,1'-biphenyl

-
B
-
34883-43-7
2,4'-dichlorobiphenyl

-
C
-
2974-90-5
3,4'-dichlorobiphenyl

-
D
-
2050-67-1
PCB 11

-
E
-
108-95-2
phenol

-
F
-
13029-08-8
2,2'-Dichlorobiphenyl

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide at 212℃; Product distribution; Mechanism; various temperatures; |

-
-
106-47-8
4-chloro-aniline

-
-
34883-43-7
2,4'-dichlorobiphenyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) NaNO2, 10percent H2SO4, 2.) TiCl3 / 1.) H2O, 0-2 deg C, 2.) H2O, MeOH, 0-5 deg C 2: NaNO2, CCl3COOH / CuCl / 2 h / 85 °C View Scheme |
-
-
138588-58-6
2,4'-dichloro-4-nitro-biphenyl

-
-
34883-43-7
2,4'-dichlorobiphenyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tin; aqueous hydrochloric acid; ethanol 2: aqueous H2SO4 / Diazotization.Behandlung der Diazoniumsalz-Loesung mit H3PO2 View Scheme |

| Conditions | Yield |
|---|---|
| With palladium diacetate; sodium carbonate In water; acetone at 25℃; for 12h; | |
| With palladium diacetate; sodium carbonate In water; acetone at 20℃; for 10h; |
-
-
29682-41-5
2,5-dichloroiodobenzene

-
A
-
34883-43-7
2,4'-dichlorobiphenyl

-
B
-
2974-90-5
3,4'-dichlorobiphenyl

-
C
-
34883-39-1
2,5-dichlorobiphenyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate; C20H29Cl2N5O2Pd / ethanol / 2.5 h / 78 - 83 °C 2: isopropyl alcohol; sodium hydroxide; palladium 10% on activated carbon / 1 h / 83 °C View Scheme |

-
-
29682-41-5
2,5-dichloroiodobenzene

-
A
-
92-52-4
biphenyl

-
B
-
34883-43-7
2,4'-dichlorobiphenyl

-
C
-
34883-39-1
2,5-dichlorobiphenyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate; C20H29Cl2N5O2Pd / ethanol / 2.5 h / 78 - 83 °C 2: isopropyl alcohol; sodium hydroxide; palladium 10% on activated carbon / 83 °C View Scheme |

-
-
694-80-4
2-bromo-1-chlorobenzene

-
-
1679-18-1
4-Chlorophenylboronic acid

-
-
34883-43-7
2,4'-dichlorobiphenyl

| Conditions | Yield |
|---|---|
| With C20H29Cl2N5O2Pd; potassium carbonate In ethanol at 78 - 83℃; for 2.5h; |
-
-
7012-37-5
2,4,4'-Trichlorobiphenyl

-
A
-
34883-43-7
2,4'-dichlorobiphenyl

-
B
-
2050-68-2
4,4'-dichlorobiphenyl

-
C
-
33284-50-3
PCB 7

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; isopropyl alcohol; sodium hydroxide at 83℃; for 1h; regioselective reaction; |

-
-
1193-72-2
1-bromo-2,4-dichlorobenzene

-
-
1679-18-1
4-Chlorophenylboronic acid

-
A
-
34883-43-7
2,4'-dichlorobiphenyl

-
B
-
2050-68-2
4,4'-dichlorobiphenyl

-
C
-
33284-50-3
PCB 7

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate; C20H29Cl2N5O2Pd / ethanol / 2.5 h / 78 - 83 °C 2: isopropyl alcohol; sodium hydroxide; palladium 10% on activated carbon / 1 h / 83 °C View Scheme |

-
-
1679-18-1
4-Chlorophenylboronic acid

-
A
-
34883-43-7
2,4'-dichlorobiphenyl

-
B
-
2974-90-5
3,4'-dichlorobiphenyl

-
C
-
34883-39-1
2,5-dichlorobiphenyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate; C20H29Cl2N5O2Pd / ethanol / 2.5 h / 78 - 83 °C 2: isopropyl alcohol; sodium hydroxide; palladium 10% on activated carbon / 1 h / 83 °C View Scheme |


| Conditions | Yield |
|---|---|
| With water Photolysis; | A 10% B 90% |

-
-
34883-43-7
2,4'-dichlorobiphenyl

| Conditions | Yield |
|---|---|
| In gas Irradiation; study of resonance enhanced two-photon ionization spectra using a time-of-flight mass spectrometer; |
-
-
34883-43-7
2,4'-dichlorobiphenyl

-
-
92-52-4
biphenyl

| Conditions | Yield |
|---|---|
| With potassium hydroxide In isopropyl alcohol at 45℃; for 20h; Inert atmosphere; | 98 %Chromat. |
2,4'-Dichloro-1,1'-biphenyl Chemical Properties
Empirical Formula: C12H8Cl2
Molecular Weight: 223.0979 g/mol
EINECS: 215-648-1
Index of Refraction: 1.594
Density: 1.249 g/cm3
Flash Point: 133.6 °C
Enthalpy of Vaporization: 52.19 kJ/mol
Boiling Point: 303.3 °C at 760 mmHg
Vapour Pressure: 0.00168 mmHg at 25 °C
Storage tempreture: 2-8 °C
Structure of 2,4'-Dichloro-1,1'-biphenyl (CAS NO.34883-43-7):
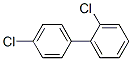
IUPAC Name: 1-Chloro-2-(4-chlorophenyl)benzene
Product Categories of 2,4'-Dichloro-1,1'-biphenyl (CAS NO.34883-43-7): Alphabetic
2,4'-Dichloro-1,1'-biphenyl Toxicity Data With Reference
| 1. | orl-mus LD50:7860 mg/kg | EVHPAZ EHP, Environmental Health Perspectives. Subseries of DHEW Publications. 24 (1978),173. |
2,4'-Dichloro-1,1'-biphenyl Safety Profile
Low toxicity by ingestion. When heated to decomposition it emits toxic vapors of Cl−.
Hazard Codes of 2,4'-Dichloro-1,1'-biphenyl (CAS NO.34883-43-7):  N
N
Risk Statements: 33-50/53
R33:Danger of cumulative effects.
R50/53:Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
Safety Statements: 35-60-61
S35:This material and its container must be disposed of in a safe way.
S60:This material and its container must be disposed of as hazardous waste.
S61:Avoid release to the environment. Refer to special instructions / safety data sheets.
2,4'-Dichloro-1,1'-biphenyl Specification
2,4'-Dichloro-1,1'-biphenyl ,its cas register number is 34883-43-7. It also can be called 2,4'-Dichlorobiphenyl ; and 1,1'-Biphenyl, 2,4'-dichloro- .
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 34884-10-1
- 34885-02-4
- 34885-03-5
- 3489-06-3
- 34892-17-6
- 3489-28-9
- 34893-50-0
- 34893-92-0
- 34896-19-0
- 34896-80-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View