-
Name
2,4-Dihydroxybenzoic acid
- EINECS 201-946-9
- CAS No. 89-86-1
- Article Data104
- CAS DataBase
- Density 1.56 g/cm3
- Solubility soluble in ethanol, diethyl ether and hot water
- Melting Point 225-227 °C
- Formula C7H6O4
- Boiling Point 414.833 °C at 760 mmHg
- Molecular Weight 154.122
- Flash Point 218.835 °C
- Transport Information
- Appearance cream to slightly beige or pink crystalline powder
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms beta-Resorcylicacid (8CI);4-Carboxyresorcinol;4-Hydroxysalicylicacid;Coupler 320;NSC 13564;NSC 4740;p-Hydroxysalicylic acid;beta-Resorcylic acid;
- PSA 77.76000
- LogP 0.79600
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogenchloride; CO2; potassium carbonate In water | 96.7% |
| With potassium dicarbonate; water | |
| With sodium hydrogencarbonate anschliessendes Einleiten von Kohlendioxid; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydroxide; CO2 In water | 95.4% |
| With CO2; potassium carbonate In water | 87.4% |

| Conditions | Yield |
|---|---|
| With sodium chlorite; sodium methylate In dimethyl sulfoxide | 95% |
| With tert.-butylhydroperoxide; copper(ll) bromide In water; acetonitrile at 20℃; for 9h; Inert atmosphere; chemoselective reaction; | 91% |
| With dihydrogen peroxide; silver nitrate In acetonitrile at 50℃; for 1h; chemoselective reaction; | 90% |
| With potassium carbonate beim Schmelzen; |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water at 20℃; for 1h; UV-irradiation; Green chemistry; regioselective reaction; | 92% |
-
-
33617-59-3
1,3-dihydroxy-4-dihydroxymethyl benzene

-
-
89-86-1
4-hydroxysalicylic acid

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; copper(ll) bromide In water; dimethyl sulfoxide for 22h; Inert atmosphere; Reflux; chemoselective reaction; | 89% |

| Conditions | Yield |
|---|---|
| With N-bromophthalimide; mercury(II) diacetate In chloroform at 20℃; for 2h; | 86% |
| Multi-step reaction with 2 steps 1: iodine 2: aq.-ethanolic NaOH-solution View Scheme | |
| With dihydrogen peroxide In water at 22 - 25℃; for 11688h; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; CO2; potassium carbonate In water | 85% |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 30℃; under 15001.5 Torr; for 28h; Solvent; Reagent/catalyst; Kolbe-Schmidt Synthesis; Autoclave; | 84% |
| With water; sodium hydrogencarbonate at 100℃; for 3h; Kolbe-Schmidt Synthesis; | 64% |
| With potassium hydrogencarbonate In water for 4h; Reflux; |

| Conditions | Yield |
|---|---|
| With iodine; dimethyl sulfoxide at 120℃; for 28h; Sealed tube; Green chemistry; | 83% |
-
-
108-24-7
acetic anhydride

-
-
89475-40-1
2,4,4'-Triacetoxy-3'-methoxy-chalkon

-
A
-
89-86-1
4-hydroxysalicylic acid

-
B
-
121-34-6
3-methoxy-4-hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With ruthenium(IV) oxide; sodium periodate 1) 5 h, room temp., pyridine; 2) acetone, water, 20 min : decomposition reaction; | A 54% B 73% |


| Conditions | Yield |
|---|---|
| With aluminium trichloride In chlorobenzene for 0.75h; Heating; | 71% |
-
-
108-24-7
acetic anhydride

-
-
4082-07-9
3'-methoxy-4',7-dihydroxyflavylium chloride

-
A
-
89-86-1
4-hydroxysalicylic acid

-
B
-
121-34-6
3-methoxy-4-hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With ruthenium(IV) oxide; sodium periodate 1) 5 h, room temp., pyridine; 2) acetone, water, 20 min : decomposition reaction; | A 48% B 64% |
| With ruthenium(IV) oxide; sodium periodate 1) 5 h, room temp., pyridine; 2) water, acetone, 20 min; Yield given. Multistep reaction. Yields of byproduct given; | |
| With ruthenium(IV) oxide; sodium periodate 1) 5 h, room temp., pyridine; 2) water, acetic ester, 20 min; Yield given. Multistep reaction. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With sodium hydroxide for 2h; Heating; | 41% |

| Conditions | Yield |
|---|---|
| In water at 160℃; under 30003 Torr; for 0.0902778h; Temperature; Time; Pressure; Kolbe-Schmidt Synthesis; | 22% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water at 20℃; for 1h; UV-irradiation; Green chemistry; | A 7% B 8% |

| Conditions | Yield |
|---|---|
| Kalischmelze; |
-
-
21392-45-0
7-hydroxycoumarin-4-carboxylic acid

-
-
89-86-1
4-hydroxysalicylic acid

| Conditions | Yield |
|---|---|
| bei der Natronschmelze; |
-
-
19829-74-4
4,6-dihydroxyisophthalic acid

-
-
89-86-1
4-hydroxysalicylic acid

| Conditions | Yield |
|---|---|
| With water at 100℃; im Rohr; |
-
-
76569-42-1
2,2,2-trichloro-1-(2,4-dihydroxy-phenyl)-ethanone

-
-
89-86-1
4-hydroxysalicylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide |
-
-
854869-04-8
9-(2,4,α-trihydroxy-benzyl)-xanthene-3,6-diol

-
-
89-86-1
4-hydroxysalicylic acid

| Conditions | Yield |
|---|---|
| at 200℃; bei der Natronschmelze; |

| Conditions | Yield |
|---|---|
| at 280℃; bei der Natronschmelze; |
-
-
35244-15-6
1-(2,4-dihydroxy-phenacyl)-pyridinium; iodide

-
-
89-86-1
4-hydroxysalicylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| Diazotization; |
-
-
109-72-8, 29786-93-4
n-butyllithium

-
-
60-29-7
diethyl ether

-
-
108-46-3
recorcinol

-
A
-
89-86-1
4-hydroxysalicylic acid

-
B
-
303-07-1
2,6-Dihydroxybenzoic acid

| Conditions | Yield |
|---|---|
| anschliessendes Behandeln mit festem CO2; |

-
-
124-41-4
sodium methylate

-
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

-
-
108-46-3
recorcinol

-
A
-
89-86-1
4-hydroxysalicylic acid

-
B
-
303-07-1
2,6-Dihydroxybenzoic acid

| Conditions | Yield |
|---|---|
| at 100℃; under 4560 Torr; |

-
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

-
-
108-46-3
recorcinol

-
-
89-86-1
4-hydroxysalicylic acid

| Conditions | Yield |
|---|---|
| With water; potassium hydrogencarbonate | |
| With water; sodium hydrogencarbonate |
-
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

-
-
108-46-3
recorcinol

-
A
-
89-86-1
4-hydroxysalicylic acid

-
B
-
303-07-1
2,6-Dihydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With sodium methylate at 100℃; under 4560 Torr; |


| Conditions | Yield |
|---|---|
| With n-butyllithium; diethyl ether anschliessend Behandeln mit Kohlendioxid; | |
| With carbon dioxide; potassium hydrogencarbonate at 120℃; | |
| With salicylic acid decarboxylase from Trichosporon moniliiforme; potassium hydrogencarbonate at 30℃; for 24h; pH=8.5; aq. phosphate buffer; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With ruthenium tetroxide; trifluoroacetic anhydride 1.) carbon tetrachloride, reflux; Yield given. Multistep reaction; | |
| With hypochlorite In aq. buffer pH=7.4; |

| Conditions | Yield |
|---|---|
| With sulfuric acid for 72h; Heating; | 100% |
| With sulfuric acid In water for 120h; Reflux; Inert atmosphere; | 100% |
| With sulfuric acid for 20h; Reflux; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate | 100% |
| With potassium hydroxide at 50℃; | |
| With potassium hydroxide | |
| With potassium carbonate In DMF (N,N-dimethyl-formamide) at 70 - 115℃; for 10.5h; | |
| With potassium carbonate In acetone |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 0.5h; | 100% |
-
-
89-86-1
4-hydroxysalicylic acid

-
-
18162-48-6
tert-butyldimethylsilyl chloride

| Conditions | Yield |
|---|---|
| Stage #1: 4-hydroxysalicylic acid With triethylamine In dichloromethane for 1h; Stage #2: tert-butyldimethylsilyl chloride In dichloromethane at 20℃; for 16h; | 100% |
-
-
89-86-1
4-hydroxysalicylic acid

-
-
616-38-6
carbonic acid dimethyl ester

-
-
2150-41-6
methyl 2, 4-dimethoxybenzoate

| Conditions | Yield |
|---|---|
| With diiron nonacarbonyl at 180℃; for 1h; Sealed tube; | 99% |

| Conditions | Yield |
|---|---|
| With alkali hydroxide In ethanol; water at 60℃; for 16h; | 98% |
| With potassium hydroxide; ethanol |
-
-
89-86-1
4-hydroxysalicylic acid

-
-
93677-89-5
5-(2,4-dichlorophenyl)-4-amino-3-mercapto-4H-1,2,4-triazole

-
-
128032-44-0
4-[3-(2,4-Dichloro-phenyl)-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazol-6-yl]-benzene-1,3-diol

| Conditions | Yield |
|---|---|
| With trichlorophosphate for 1h; Heating; | 98% |
| With trichlorophosphate for 1h; Heating; | 98% |
-
-
89-86-1
4-hydroxysalicylic acid

-
-
100-39-0
benzyl bromide

-
-
121903-70-6
benzyl 2,4-bis(benzyloxy)benzoate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20 - 90℃; for 13h; Inert atmosphere; | 98% |
| With potassium carbonate In acetonitrile at 70℃; for 5h; | 98% |
| With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; for 16h; | 77% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20 - 50℃; | 96% |
| With sodium hydroxide; sodium hydride Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In acetonitrile Reflux; | 96% |
| With dicyclohexyl-carbodiimide Reflux; |

| Conditions | Yield |
|---|---|
| With aluminum oxide; methanesulfonic acid at 80℃; for 0.25h; | 95% |

| Conditions | Yield |
|---|---|
| With bromine; acetic acid at 20℃; regioselective reaction; | 94% |
| With bromine; acetic acid at 20℃; regioselective reaction; | 94% |
| With bromine; acetic acid | 90% |

| Conditions | Yield |
|---|---|
| With potassium hydrogencarbonate In N,N-dimethyl-formamide at 40℃; | 94% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile for 24h; Ambient temperature; | 85% |
| Stage #1: 4-hydroxysalicylic acid With sodium hydrogencarbonate In N,N-dimethyl-formamide at 20℃; Stage #2: methyl iodide In N,N-dimethyl-formamide at 40℃; for 10h; | 84.4% |

| Conditions | Yield |
|---|---|
| With pyridine; dmap at 20℃; for 24h; | 93% |
| With dmap; triethylamine In tetrahydrofuran at 20℃; for 4h; | 93% |
| With sulfuric acid at 65℃; for 0.5h; | 80% |

| Conditions | Yield |
|---|---|
| With Eaton′s Reagent at 80℃; for 1h; | 92% |
| With zinc(II) chloride; trichlorophosphate at 75℃; Microwave irradiation; | 84% |
| With tin(ll) chloride at 140℃; for 0.0138889h; Microwave irradiation; regioselective reaction; | 81% |
-
-
89-86-1
4-hydroxysalicylic acid

-
-
856106-63-3
2,4-dihydroxy-5-iodobenzoic acid

| Conditions | Yield |
|---|---|
| With Iodine monochloride; acetic acid at 20℃; for 4h; | 92% |
| With Iodine monochloride In acetic acid at 20℃; for 5h; | 91% |
| With Iodine monochloride; acetic acid for 4h; | 69% |
| With diethyl ether; Iodine monochloride | |
| With Iodine monochloride In acetic acid at 20℃; |

| Conditions | Yield |
|---|---|
| With potassium hydrogencarbonate In N,N-dimethyl-formamide at 40℃; | 92% |
| With potassium hydrogencarbonate In N,N-dimethyl-formamide at 20℃; for 5h; | 83% |
| With sodium hydrogencarbonate In N,N-dimethyl-formamide at 90℃; for 7h; | 70% |
| In N,N-dimethyl-formamide |

| Conditions | Yield |
|---|---|
| With sulfuric acid for 15h; Reflux; | 92% |
-
-
89-86-1
4-hydroxysalicylic acid

-
-
57857-70-2
di-(p-methoxyphenyl)tellurium oxide

-
-
84438-49-3
Di(p-anizyl)tellurium diresorcylate

| Conditions | Yield |
|---|---|
| In propan-1-ol | 91% |
-
-
89-86-1
4-hydroxysalicylic acid

-
A
-
7355-22-8
5-bromo-4-hydroxysalicylic acid

-
B
-
3147-46-4
3,5-dibromo-4-hydroxysalicylic acid

| Conditions | Yield |
|---|---|
| With bromine In acetic acid at 25℃; for 4h; | A 91% B 5% |
| With bromine In acetic acid at 25℃; for 5h; | A 75% B n/a |
-
-
89-86-1
4-hydroxysalicylic acid

-
-
57438-38-7
2,4-dihydroxybenzoyl chloride

| Conditions | Yield |
|---|---|
| With thionyl chloride In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; for 8.5h; | 90.4% |
| With thionyl chloride | |
| With thionyl chloride In chloroform Heating; | |
| With thionyl chloride for 4h; Chlorination; Heating; | |
| With thionyl chloride |

| Conditions | Yield |
|---|---|
| In benzene at -20℃; for 6h; | 90.1% |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 0℃; Reflux; Inert atmosphere; | 90% |
| With thionyl chloride Inert atmosphere; Reflux; | 62% |
| With sulfuric acid for 18h; Reflux; | 57% |

| Conditions | Yield |
|---|---|
| With alumina sulfuric acid at 110℃; for 3h; | 90% |
| With hydrogenchloride Heating; | |
| With trichlorophosphate In o-xylene Heating; | |
| With sulfuric acid Fischer-Speier Esterification; |
-
-
89430-08-0
C20H15N2PS2

-
-
89-86-1
4-hydroxysalicylic acid

| Conditions | Yield |
|---|---|
| In dichloromethane | 90% |

| Conditions | Yield |
|---|---|
| With triethylamine In acetone | 90% |

2,4-Dihydroxybenzoic acid Consensus Reports
2,4-Dihydroxybenzoic acid Specification
The 2,4-Dihydroxybenzoic acid, with the CAS registry number 89-86-1, is also known as beta-Resorcylic acid. It belongs to the product categories of Intermediates of Dyes and Pigments; Fine Chemical & Intermediates; Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts; Benzoic Acid Derivative; Organic acids; Building Blocks; C7; Carbonyl Compounds; Carboxylic Acids; Chemical Synthesis; Organic Building Blocks. Its EINECS number is 201-946-9. This chemical's molecular formula is C7H6O4 and molecular weight is 154.12. What's more, its systematic name is 2,4-Dihydroxybenzoic acid. Its classification code is Reproductive Effect. This chemical should be sealed and stored in a cool and dry place. It is used as an intermediate for dyestuffs and drugs. is a dihydroxybenzoic acid. As a resorcyclic acid, it is one of the three isomeric crystalline acids that are both carboxyl derivatives of resorcinol and dihydroxy derivatives of benzoic acid. It is a degradation product of cyanidin glycosides from tart cherries in cell cultures. It is also a metabolite found in human plasma after cranberry juice consumption.
Physical properties of 2,4-Dihydroxybenzoic acid are: (1)ACD/LogP: 1.765; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -1.08; (4)ACD/LogD (pH 7.4): -1.39; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 1.00; (8)ACD/KOC (pH 7.4): 1.00; (9)#H bond acceptors: 4; (10)#H bond donors: 3; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 77.76 Å2; (13)Index of Refraction: 1.671; (14)Molar Refractivity: 36.946 cm3; (15)Molar Volume: 98.823 cm3; (16)Polarizability: 14.647×10-24cm3; (17)Surface Tension: 84.3 dyne/cm; (18)Density: 1.56 g/cm3; (19)Flash Point: 218.835 °C; (20)Enthalpy of Vaporization: 70.412 kJ/mol; (21)Boiling Point: 414.833 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
Preparation: this chemical can be prepared by 2,4-dihydroxy-benzaldehyde. This reaction will need reagents NaClO2, CH3ONa and solvent dimethylsulfoxide. The yield is about 95%.

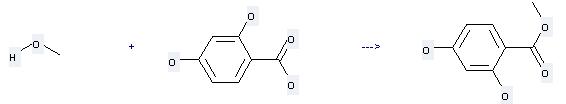
Uses of 2,4-Dihydroxybenzoic acid: it can be used to produce 2,4-dihydroxy-benzoic acid methyl ester by heating. It will need reagent H2SO4 with the reaction time of 72 hours. The yield is about 100%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing, gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)SMILES: c1cc(c(cc1O)O)C(=O)O
(2)Std. InChI: InChI=1S/C7H6O4/c8-4-1-2-5(7(10)11)6(9)3-4/h1-3,8-9H,(H,10,11)
(3)Std. InChIKey: UIAFKZKHHVMJGS-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | > 800mg/kg (800mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 196, Pg. 478, 1976. |
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 898656-60-5
- 89866-61-5
- 89868-06-4
- 89868-13-3
- 89-87-2
- 898746-54-8
- 898746-82-2
- 898747-55-2
- 898747-71-2
- 898747-75-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View