-
Name
3',4'-Anhydrovinblastine
- EINECS
- CAS No. 38390-45-3
- Article Data19
- CAS DataBase
- Density 1.35 g/cm3
- Solubility
- Melting Point 205-207oC
- Formula C46H56N4O8
- Boiling Point
- Molecular Weight 792.973
- Flash Point
- Transport Information
- Appearance white crystal power
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms (+)-Anhydrovinblastine;3',4'-Dehydroisoleurosine;3',4'-Dehydrovinblastine;3',4'-Didehydroisoleurosine;3',4'-Didehydrovinblastine;Anhydrovincaleukoblastine;F 81097;Anhydrovinblastine;
- PSA 133.87000
- LogP 4.73690
3',4'-Anhydrovinblastine Chemical Properties
Molecular Formula: C46H56N4O8
Molar mass: 792.9588 g/mol
Density: 1.67
Index of Refraction: 1.67
Structure of Anhydrovinblastine (38390-45-3):
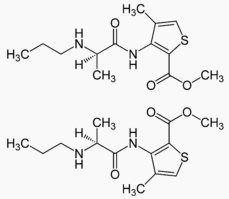
XLogP3-AA: 4
H-Bond Donor: 2
H-Bond Acceptor: 11
Canonical SMILES: CCC1=CC2CC(C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7C(C=CC9)(C(C(C8N6C)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC
Isomeric SMILES: CCC1=C[C@@H]2C[C@@](C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)[C@]
78CCN9[C@H]7[C@@](C=CC9)([C@H]([C@@]([C@@H]8N6C)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC
InChI: InChI=1S/C46H56N4O8/c1-8-28-21-29-24-45(41(52)56-6,
37-31(15-19-49(25-28)26-29)30-13-10-11-14-34(30)47-37)33-22-32-35(23-36
(33)55-5)48(4)39-44(32)17-20-50-18-12-16-43(9-2,38(44)50)40(58-27(3)51)46(39,54)42(53)57-7/h10-14,16,21-23,29,38-40,47,54H,8-9,15,17-20,24-26H2,1-7H3/t29-,38+,39-,40-,43-,44-,45+,46+/m1/s1
InChIKey: FFRFGVHNKJYNOV-DOVUUNBWSA-N
3',4'-Anhydrovinblastine History
Anhydrovinblastine (38390-45-3) was first synthetized by Rusching in 1969, and brought to the market in Germany by Hoechst AG under the brand name Ultracain.In 1983 it was brought into the North American market, to Canada, under the name Ultracaine for dental use. This brand is currently manufactured in Germany by Sanofi-Aventis and distributed in North America by Hansamed Limited(since 1999).It was approved by the FDA in April 2000, and became available in the United States of America two months later under the brand name Septocaine with Epinephrine 1:100,000 (Septodont).Articadent became available in the United States in October of 2010.Articaine is currently available for the North American dental market.
3',4'-Anhydrovinblastine Specification
Anhydrovinblastine (38390-45-3) , also can be called for 3',4'-Anhydrovinblastine ; 3',4'-Didehydro-4'-deoxyvincaleukoblastine ; 3',4'-Dehydroisoleurosine , is a dental local anesthetic. It is the most widely used local anesthetic in a number of European countries and is available in many countries around the world. Anhydrovinblastine (38390-45-3), In dentistry, is used both for infiltration and block injections. And it is used for pain control.
It is not contraindicated in patients with sulfa allergies; there is no cross-allergenicity between articaine's sulfer-bearing thiophene ring and sulfonamides.
Related Products
- 3,10-Diaminotricyclo(5.2.1.0(sup 2,6))decane
- 3,10-Dinitrophenanthrene
- 3-((10-ETHYL-11-(p-HYDROXYPHENYL)DIBENZ-(B,F)OXEPIN-3-YL)OXY)-1,2-PROPANEDIOL HYDRATE (4:1)
- 3-(1,1,2,2-Tetrafluoroethoxy)aniline
- 3-(1,1,2,2-Tetrafluoroethoxy)benzaldehyde
- 3-(1,1,2,2-Tetrafluoroethoxy)bromobenzene
- 3-(1,1,2,2-Tetrafluoroethoxy)toluene
- 3-[1,1'-Biphenyl]-4-yl-1,2,3,4-tetrahydro-1-naphthol
- 3,11-Dichloro-6,11-dihydro-6-methyldibenzo[c,f][1,2]thiazepine 5,5-dioxide
- 3-[(1,1-Dimethyl-2-hydroxyethyl)amino]-2-hydroxypropanesulfonicacid
- 383907-17-3
- 3839-22-3
- 38395-02-7
- 38395-64-1
- 38396-89-3
- 38399-65-4
- 38400-84-9
- 38401-71-7
- 3840-18-4
- 3840-27-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View