-
Name
4'-METHYL-2,2'-BIPYRIDINE-4-CARBOXYLIC ACID
- EINECS 145-896-5
- CAS No. 103946-54-9
- Article Data34
- CAS DataBase
- Density 1.26 g/cm3
- Solubility
- Melting Point 280 °C
- Formula C12H10N2O2
- Boiling Point 497.4 °C at 760 mmHg
- Molecular Weight 214.224
- Flash Point 254.6 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 4-Carboxy-4'-methyl-2,2'-bipyridine;4-Methyl-2,2'-bipyridine-4'-carboxylic acid;
- PSA 63.08000
- LogP 2.15020
Synthetic route
-
-
1134-35-6
4,4'-dimethyl-2,2'-bipyridines

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide; sulfuric acid | 98% |
| Stage #1: 4,4'-dimethyl-2,2'-bipyridines With selenium(IV) oxide In 1,4-dioxane for 30h; Reflux; Stage #2: With silver nitrate; sodium hydroxide In ethanol; water for 24h; | 77% |
| Stage #1: 4,4'-dimethyl-2,2'-bipyridines With selenium(IV) oxide In 1,4-dioxane for 16h; Oxidation; Heating; Stage #2: With silver(l) oxide Oxidation; | 72% |
-
-
104704-09-8
4'-methyl-2,2'-bipyridine-4-carboxylaldehyde

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With silver nitrate; sodium hydroxide In ethanol for 24h; Darkness; | 82% |
| With silver nitrate; sodium hydroxide In ethanol; water at 20℃; | 80% |
| Stage #1: 4'-methyl-2,2'-bipyridine-4-carboxylaldehyde With silver nitrate In ethanol for 0.333333h; Stage #2: With sodium hydroxide In ethanol at 25℃; for 15h; Darkness; | 80% |
-
-
1134-35-6
4,4'-dimethyl-2,2'-bipyridines

-
A
-
6813-38-3
2,2'-Bipyridine-4,4'-dicarboxylic acid

-
B
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With potassium permanganate; sulfuric acid for 12h; Heating; | A 45% B 4% |
| With manganese(II) sulfate; potassium permanganate In pyridine; water for 120h; Ambient temperature; | A n/a B 7% |
| With sodium hydroxide; selenium(IV) oxide; silver nitrate 1.) 1,4-dioxane, reflux, 24 h, 2.) EtOH, water, 15 h; Yield given. Multistep reaction. Yields of byproduct given; | |
| With chromium(VI) oxide; periodic acid In dichloromethane; acetonitrile at 20℃; for 16h; Overall yield = 0.099 g; | A 35 %Spectr. B 6 %Spectr. |
-
-
1134-35-6
4,4'-dimethyl-2,2'-bipyridines

-
A
-
104704-09-8
4'-methyl-2,2'-bipyridine-4-carboxylaldehyde

-
B
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide In 1,4-dioxane for 72h; | A 29% B n/a |
-
-
108-89-4
picoline

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: manganese(IV) oxide; palladium on activated charcoal / neat (no solvent) / 168 h / 140 °C 2.1: selenium(IV) oxide / 1,4-dioxane / 24 h / Reflux 2.2: 20 °C View Scheme |
-
-
1134-35-6
4,4'-dimethyl-2,2'-bipyridines

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide; sulfuric acid | 98% |
| Stage #1: 4,4'-dimethyl-2,2'-bipyridines With selenium(IV) oxide In 1,4-dioxane for 30h; Reflux; Stage #2: With silver nitrate; sodium hydroxide In ethanol; water for 24h; | 77% |
| Stage #1: 4,4'-dimethyl-2,2'-bipyridines With selenium(IV) oxide In 1,4-dioxane for 16h; Oxidation; Heating; Stage #2: With silver(l) oxide Oxidation; | 72% |
-
-
104704-09-8
4'-methyl-2,2'-bipyridine-4-carboxylaldehyde

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With silver nitrate; sodium hydroxide In ethanol for 24h; Darkness; | 82% |
| With silver nitrate; sodium hydroxide In ethanol; water at 20℃; | 80% |
| Stage #1: 4'-methyl-2,2'-bipyridine-4-carboxylaldehyde With silver nitrate In ethanol for 0.333333h; Stage #2: With sodium hydroxide In ethanol at 25℃; for 15h; Darkness; | 80% |
-
-
1134-35-6
4,4'-dimethyl-2,2'-bipyridines

-
A
-
6813-38-3
2,2'-Bipyridine-4,4'-dicarboxylic acid

-
B
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With potassium permanganate; sulfuric acid for 12h; Heating; | A 45% B 4% |
| With manganese(II) sulfate; potassium permanganate In pyridine; water for 120h; Ambient temperature; | A n/a B 7% |
| With sodium hydroxide; selenium(IV) oxide; silver nitrate 1.) 1,4-dioxane, reflux, 24 h, 2.) EtOH, water, 15 h; Yield given. Multistep reaction. Yields of byproduct given; | |
| With chromium(VI) oxide; periodic acid In dichloromethane; acetonitrile at 20℃; for 16h; Overall yield = 0.099 g; | A 35 %Spectr. B 6 %Spectr. |
-
-
1134-35-6
4,4'-dimethyl-2,2'-bipyridines

-
A
-
104704-09-8
4'-methyl-2,2'-bipyridine-4-carboxylaldehyde

-
B
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide In 1,4-dioxane for 72h; | A 29% B n/a |
-
-
108-89-4
picoline

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: manganese(IV) oxide; palladium on activated charcoal / neat (no solvent) / 168 h / 140 °C 2.1: selenium(IV) oxide / 1,4-dioxane / 24 h / Reflux 2.2: 20 °C View Scheme |
-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

-
-
110-70-3
N,N`-dimethylethylenediamine

-
-
524957-05-9
4'-methyl-[2,2']bipyridinyl-4-carboxylic acid methyl-(2-methylamino-ethyl)-amide

| Conditions | Yield |
|---|---|
| Stage #1: 4'-methyl-2,2'-bipyridine-4-carboxylic acid With 1,1'-carbonyldiimidazole In tetrahydrofuran at 60℃; Stage #2: N,N`-dimethylethylenediamine In tetrahydrofuran at 20℃; | 100% |

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4'-methyl-2,2'-bipyridine-4-carboxylic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: meso-tetrakis-[4-(aminoethoxy-ethoxyethyl-aminocarbonyl) phenyl] porphyrin With dmap In N,N-dimethyl-formamide for 24h; Darkness; | 96% |

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With fluorophosphate; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; | 95% |
-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

-
-
62-53-3
aniline

-
-
1447716-06-4
4-methyl-N-phenyl-(2,2'-bipyridine)-4-carboxamide

| Conditions | Yield |
|---|---|
| Stage #1: 4'-methyl-2,2'-bipyridine-4-carboxylic acid With thionyl chloride for 2h; Reflux; Stage #2: aniline With triethylamine In tetrahydrofuran at 60℃; | 95% |

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With sodium acetate; hydrochloric acid In methanol; water soln. Ru complex, ligand, and NaOAc in aq. MeOH was heated to reflux for24 h; soln. was cooled to room temp., concd., adjusted to pH 1 by HCl, aq. NaCl was added, ppt. was filtered, dissolved in MeOH and evapd., residue was dissolved in CH2Cl2 and filtered, benzene was added, ppt. was filtered, washed, dried in vacuo; elem. anal.; | 92% |

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| In ethanol soln. of Ru complex in EtOH stirred under N2 for 10 min; 0.5 equiv. of ligand added; refluxed for 5 h; cooled to 25°C; satd. aq. soln. of NH4PF6 added; filtered; | 91% |

-
-
17084-13-8
potassium hexafluorophosphate

-
-
221362-55-6, 41060-99-5
[Ru(2,2'-bipyridine)(CO)Cl2]2

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| In 2-methoxy-ethanol; water bipyridine was added to soln. of Ru complex in methoxyethanol under N2; refluxed at 120°C for 2 h; evapd. (vac.); H2O was added; sonicated for 5 min; cooled to 4°C; filtered; mixed with aq. KPF6; filtered; washed (H2O, H2O/EtOH, 1/1); dried at 70°C in air; elem. anal.; | 91% |

-
-
22112-84-1
tetrakis(4-aminophenyl)porphyrin

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4'-methyl-2,2'-bipyridine-4-carboxylic acid With pyridine; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride for 0.166667h; Stage #2: tetrakis(4-aminophenyl)porphyrin at 20℃; for 3h; | 91% |
-
-
12354-84-6, 12354-85-7
bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]; 4'-methyl-2,2'-bipyridine-4-carboxylic acid In methanol for 18h; Stage #2: ammonium hexafluorophosphate at 3.84℃; for 18h; | 91% |


-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With NaH2PO4; Na2HPO4 In ethanol; water Ru complex and ligand (molar ratio 1:1.2) refluxed in EtOH-H2O for 17 h; dissolved in MeCN; deionized water (NaH2PO4-Na2HPO4 buffer) added slowly; filtered; sepd. by cation-exchange chromy. (Sephadex, NaCl soln.); pptd. by adding excess of NH4PF6 in H2O; acidified with dilute HCl; cooled to 0°C for 2 h; filtered; rinsed with dilute aq. NH4PF6 (acidic) and Et2O; elem. anal.; | 90% |


| Conditions | Yield |
|---|---|
| Stage #1: 4'-methyl-2,2'-bipyridine-4-carboxylic acid With benzotriazol-1-ol; dicyclohexyl-carbodiimide In tetrahydrofuran; N,N-dimethyl-formamide for 0.5h; Cooling with ice; Stage #2: C36H45N7O10S*ClH In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; for 14h; pH=8 - 9; | 89% |

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| In methanol; dichloromethane suspn. of Ir complex in MeOH added to suspn. of ligand in CH2Cl2; heatedto reflux with stirring for 2 h; cooled to room temp.; satd. soln. of N H4PF6 in MeOH added; stirred for 30 min; vol. reduced under vac.; filtered; ppt. washed with ether; extd. into CH2Cl2; solvent removed under reduced pressure; elem. anal.; | 88% |


-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With NaOAc; H2SO4 In water; ethylene glycol Ru complex, ligand, NaOAc, H2O and ethylene glycol heated at 120°C overnight, concd., H2SO4 added (to pH=1), H2O added, filtered, pptd. with aq. NH4PF6; filtered, washed with water, dried in vac.; | 85% |
| With sodium acetate; ethylene glycol; H2SO4 In water Ru-contg. compd. (0.419 mmol), a ligand (1 mmol), NaCH3CO2 (2.7 mmol), H2O, and ethylene glycol were heated at 120°C for 1 night; pH = 1 (H2SO4); H2O was added; the mixt. was filtered; addn. of satd. aq. soln.of NH4PF6; the complex was filtered, washed with H2O and dried under vac.; | 85% |


-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With NaOAc; H2SO4 In water; ethylene glycol Ru complex, ligand, NaOAc, H2O and ethylene glycol heated at 120°C overnight, concd., H2SO4 added (to pH=1), H2O added, filtered, pptd. with aq. NH4PF6; filtered, washed with water, dried in vac.; | 85% |
| With sodium acetate; ethylene glycol; H2SO4 In water Ru-contg. compd. (0.419 mmol), a ligand (1 mmol), NaCH3CO2 (2.7 mmol), H2O, and ethylene glycol were heated at 120°C for 1 night; pH = 1 (H2SO4); H2O was added; the mixt. was filtered; addn. of satd. aq. soln.of NH4PF6; the complex was filtered, washed with H2O and dried under vac.; | 85% |


-
-
33915-80-9
chloridobis(2-phenyl-pyridine)rhodium(III) dimer

-
-
7732-18-5
water

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| In methanol; dichloromethane soln. of Rh complex in CH2Cl2 added to suspn. of ligand in MeOH; heated to reflux with stirring for 3 h; cooled to room temp.; satd. soln. of NH4PF6 in MeOH added; stirred for 30 min; vol. reduced under vac.; filtered; ppt. washed with ether; extd. into acetone; solvent removed under reduced pressure; extd. into CH2Cl2; filtered; elem. anal.; | 85% |


-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4'-methyl-2,2'-bipyridine-4-carboxylic acid With 4-methyl-morpholine; 2-chloro-4,6-dimethoxy-1 ,3,5-triazine In N,N-dimethyl-formamide at 0℃; for 4h; Stage #2: C51H50N9O3(3+)*3I(1-)*3C2HF3O2 In N,N-dimethyl-formamide at 20℃; for 24h; Darkness; | 85% |
-
-
345911-20-8, 19542-80-4, 158060-65-2, 34795-02-3, 15746-57-3
cis-dichlorobis(2,2′-bipyridine)ruthenium(II)

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: cis-dichlorobis(2,2′-bipyridine)ruthenium(II); 4'-methyl-2,2'-bipyridine-4-carboxylic acid In ethanol; water for 5h; Reflux; Darkness; Inert atmosphere; Stage #2: ammonium hexafluorophosphate In water | 85% |
| Stage #1: cis-dichlorobis(2,2′-bipyridine)ruthenium(II); 4'-methyl-2,2'-bipyridine-4-carboxylic acid In methanol; water for 2h; Reflux; Inert atmosphere; Stage #2: ammonium hexafluorophosphate In water | 74% |
| Stage #1: cis-dichlorobis(2,2′-bipyridine)ruthenium(II) In methanol Inert atmosphere; Schlenk technique; Reflux; Stage #2: 4'-methyl-2,2'-bipyridine-4-carboxylic acid Inert atmosphere; Schlenk technique; Reflux; Stage #3: ammonium hexafluorophosphate Inert atmosphere; Schlenk technique; |

-
-
345911-20-8, 19542-80-4, 158060-65-2, 34795-02-3, 15746-57-3
cis-dichlorobis(2,2′-bipyridine)ruthenium(II)

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: cis-dichlorobis(2,2′-bipyridine)ruthenium(II); 4'-methyl-2,2'-bipyridine-4-carboxylic acid With n-butylamine hydrochloride In ethanol for 5h; Reflux; Inert atmosphere; Darkness; Stage #2: In acetone; acetonitrile | 85% |
-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

-
-
99532-86-2
N,2-[(1,1-dimethylethoxy)carbonyl]-L-lysine methyl ester hydrochloride

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; dmap; (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; 1-hydroxybenzotriazol-hydrate In N,N-dimethyl-formamide for 24h; Ambient temperature; | 84% |
-
-
51857-17-1
tert-butyl N-(6-aminohexyl)carbamate

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

-
-
1186077-56-4
tert-butyl (6-(4’-methyl-[2,2’-bipyridine]-4-carboxamido)hexyl)carbamate

| Conditions | Yield |
|---|---|
| Stage #1: 4'-methyl-2,2'-bipyridine-4-carboxylic acid With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 60℃; for 0.5h; Inert atmosphere; Stage #2: tert-butyl N-(6-aminohexyl)carbamate In N,N-dimethyl-formamide at 60℃; for 16h; Inert atmosphere; | 83% |
| With dmap; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane Inert atmosphere; | 51.43% |

-
-
106548-41-8, 889112-21-4
[(4,7-diphenyl-1,10-phenanthroline)2RuCl2]

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: [(4,7-diphenyl-1,10-phenanthroline)2RuCl2]; 4'-methyl-2,2'-bipyridine-4-carboxylic acid In ethanol; water Inert atmosphere; Reflux; Stage #2: ammonium hexafluorophosphate In water | 83% |


-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| In ethanol; water suspn. of Ru complex and ligand in 70% aq. EtOH heated under reflux overnight, cooled to room temp., concd. (vac.), stored overnight, filtered, solid washed with H2O, combined filtrates acidified to pH 1 by dropwise addn. of 60% aq. HPF6 with stirring; ppt. filtered off, washed with H2O and diethyl ether, dried at 110°C overnight; elem. anal.; | 82% |


-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| In ethanol; water suspn. of Ru complex and ligand in 70% aq. EtOH heated under reflux overnight, cooled to room temp., concd. (vac.), stored overnight, filtered, solid washed with H2O, combined filtrates acidified to pH 1 by dropwise addn. of 60% aq. HPF6 with stirring; ppt. filtered off, washed with H2O and diethyl ether, dried at 110°C overnight; elem. anal.; | 82% |

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

-
-
67605-64-5
5-(p-aminophenyl)-10,15,20-triphenylporphine

| Conditions | Yield |
|---|---|
| Stage #1: 4'-methyl-2,2'-bipyridine-4-carboxylic acid With pyridine; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride for 0.166667h; Stage #2: 5-(p-aminophenyl)-10,15,20-triphenylporphine at 20℃; for 1h; | 82% |
-
-
134812-30-9
N-(4-(3'-aminophenyl)thiazol-2-yl)acetamide

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With dmap; 1-hydroxy-7-aza-benzotriazole; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 6h; | 82% |
-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

-
-
191601-42-0
N4,N4'-Dimethyl-N4-(4'-methyl-[2,2']bipyridinyl-4-ylmethyl)-biphenyl-4,4'-diamine

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; bromo-tris(1-pyrrolidinyl)phosphonium hexafluorophosphate In dichloromethane Ambient temperature; | 80% |

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: cis-dichloridobis(1,10-phenanthroline)ruthenium(II); 4'-methyl-2,2'-bipyridine-4-carboxylic acid In ethanol for 12h; Reflux; Stage #2: hexafluorophosphoric acid With ammonium hexafluorophosphate pH=1; | 80% |

-
-
67-56-1
methanol

-
-
103946-54-9
4'-methyl-2,2'-bipyridine-4-carboxylic acid

-
-
142593-05-3
methyl 4'-methyl-2,2'-bipyridine-4-carboxylate

| Conditions | Yield |
|---|---|
| With sulfuric acid at 0℃; Inert atmosphere; Reflux; | 79% |
4'-Methyl-2,2'-bipyridine-4-carboxylic acid Chemical Properties
Molecular Formula: C12H10N2O2
Molecular Weight: 214.22 g/mol
Index of Refraction: 1.608
Density: 1.26 g/cm3
Flash Point: 254.6 °C
Enthalpy of Vaporization: 80.61 kJ/mol
Boiling Point: 497.4 °C at 760 mmHg
Vapour Pressure: 1.03E-10 mmHg at 25 °C
Structure of 4'-Methyl-2,2'-bipyridine-4-carboxylic acid (CAS NO.103946-54-9):
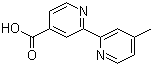
Product Category of 4'-Methyl-2,2'-bipyridine-4-carboxylic acid (CAS NO.103946-54-9): API intermediates
4'-Methyl-2,2'-bipyridine-4-carboxylic acid Specification
4'-Methyl-2,2'-bipyridine-4-carboxylic acid (CAS NO.103946-54-9) also can be called [2,2'-bipyridine]-4-carboxylic acid, 4'-methyl- .
Related Products
- 4-Methyl-2-(2'-nitrophenyl)azophenol
- 4-Methyl-2-(3-pyridinyl)-1,3-thiazole-5-carboxylic acid
- 4-Methyl-2-(4-(trifluoromethyl)phenyl)thiazole-5-carboxylic acid
- 4-Methyl-2(5H)-furanone
- 4-Methyl-2-(methylamino)-1,3-thiazole-5-carboxylic acid
- 4-Methyl-2-(methylimino)-1-imidazolidinamine
- 4-Methyl-2-(methylthio)pyrimidine
- 4-Methyl-2,5-diphenylpyridine
- 4-Methyl-2,6,7-trioxa-1-arsabicyclo(2.2.2)octane
- 4-Methyl-2,6-bis(1-phenylethyl)-phenol
- 103949-58-2
- 103951-51-5
- 103956-00-9
- 103956-09-8
- 103-95-7
- 103957-56-8
- 103957-83-1
- 10396-10-8
- 103962-05-6
- 103962-10-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View