-
Name
Acid Orange 7
- EINECS 211-199-0
- CAS No. 633-96-5
- Article Data3
- CAS DataBase
- Density 1.525[at 20℃]
- Solubility 116 g/L (30 °C) in water
- Melting Point 164 °C
- Formula C16H11N2NaO4S
- Boiling Point
- Molecular Weight 350.33
- Flash Point
- Transport Information
- Appearance Orange-red powder
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Acid Orange A (6CI);Benzenesulfonic acid, 4-[(2-hydroxy-1-naphthalenyl)azo]-,monosodium salt (9CI);Benzenesulfonic acid, p-(2-hydroxy-1-naphthylazo)-, sodium salt (6CI);11550Orange;AO 7;Acid LeatherOrange extra;Acid Orange II;Amacid Orange Y Conc;BTK Orange II;Basacid Orange 280;Betanaphtholorange;Borunil Orange A 2R;Bucacid Orange A;C Ext. Orange8;C.I. 15510;Calcocid Orange Y;Certiqual Orange II;Colocid Orange II;Conacid Orange L;Covalene Orange II;CurolOrange;D and C Orange 4 Aluminum Lake;Daedo Acid Orange 2G;Derma Fur Orange R 125;Diacid Orange II;Dyacid Orange II;Dycosacid Orange Yellow II;Eniacid OrangeII;Erio Orange II;Fenazo Orange;Japan Orange 205;Japan Orange No. 205;Java Orange II;Kromon Lake Orange Toner;Lake Orange A;Libacid Orange LII;Lurazol Orange E;Multicuer Orange II;Naphthalene Orange G;Naphthol RedJ;Naphtocard Orange II;Neklacid Orange II;No. 177 OrangeLake;Nubilon Orange R;Orange 2;Orange II;
- PSA 110.53000
- LogP 4.94570
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: 4-aminobenzene sulfonic acid With sodium nitrite In water; N,N-dimethyl-formamide at 25℃; Stage #2: With hydrogenchloride In water; N,N-dimethyl-formamide Stage #3: β-naphthol In water; N,N-dimethyl-formamide at 25℃; for 0.00805556h; | 94.74% |
| Stage #1: 4-aminobenzene sulfonic acid With water; sodium nitrite In neat (no solvent) at 20℃; Stage #2: β-naphthol In neat (no solvent) at 20℃; for 0.333333h; | 83% |
| With sodium nitrite at 0℃; Neat (no solvent); | 25% |

| Conditions | Yield |
|---|---|
| In water for 0.25h; | 97% |

| Conditions | Yield |
|---|---|
| In water for 0.25h; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: orange II With nitric acid In water Inert atmosphere; Stage #2: silver carbonate In water at 60℃; for 0.5h; Temperature; Inert atmosphere; Darkness; | 88.4% |

| Conditions | Yield |
|---|---|
| In water; acetic acid pH=2.6; |

| Conditions | Yield |
|---|---|
| With sodium disulfite; borohydride at 25℃; pH=5.6; Reduction; | |
| With iron In various solvent(s) pH=7.0; Kinetics; Reduction; |
-
-
633-96-5
orange II

| Conditions | Yield |
|---|---|
| at 5℃; |

-
-
633-96-5
orange II

-
A
-
116-63-2
1-amino-2-naphthol-4-sulfonic acid

-
B
-
121-57-3
4-aminobenzene sulfonic acid

| Conditions | Yield |
|---|---|
| bei folgendem Ansaeuern mit Salzsaeure; |

-
-
633-96-5
orange II

| Conditions | Yield |
|---|---|
| beim Zufuegen von Phenylhydrazin und weiteren Erwaermen; |


| Conditions | Yield |
|---|---|
| With ethylenediaminetetraacetic acid; dihydrogen peroxide; ferric nitrate In water for 0.166667h; pH=7; |

| Conditions | Yield |
|---|---|
| In chloroform; water pH=5; aq. acetate buffer; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium dithionite / 40 - 50 °C 2: iron(III) chloride; hydrogenchloride / Cooling with ice 3: sodium azide / water; acetic acid / 2 h / 40 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: sodium dithionite / 40 - 50 °C 1.2: Cooling with ice 2.1: sodium azide; acetic acid / water / 40 °C View Scheme |
-
-
633-96-5
orange II

-
-
1386994-19-9
4-(4-(dimethylamino)benzylideneamino)naphthalene-1,2-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium dithionite / 40 - 50 °C 2: iron(III) chloride; hydrogenchloride / Cooling with ice 3: sodium azide / water; acetic acid / 2 h / 40 °C 4: acetic acid / ethanol; methanol / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium dithionite / 40 - 50 °C 1.2: Cooling with ice 2.1: sodium azide; acetic acid / water / 40 °C 3.1: acetic acid / ethanol / Reflux View Scheme |
-
-
633-96-5
orange II

-
-
1386994-20-2
4-(3,4,5-trimethoxybenzylideneamino)naphthalene-1,2-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium dithionite / 40 - 50 °C 2: iron(III) chloride; hydrogenchloride / Cooling with ice 3: sodium azide / water; acetic acid / 2 h / 40 °C 4: acetic acid / ethanol; methanol / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium dithionite / 40 - 50 °C 1.2: Cooling with ice 2.1: sodium azide; acetic acid / water / 40 °C 3.1: acetic acid / ethanol / Reflux View Scheme |
-
-
633-96-5
orange II

-
-
1386994-21-3
4-(3,4-dimethoxybenzylideneamino)naphthalene-1,2-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium dithionite / 40 - 50 °C 2: iron(III) chloride; hydrogenchloride / Cooling with ice 3: sodium azide / water; acetic acid / 2 h / 40 °C 4: acetic acid / ethanol; methanol / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium dithionite / 40 - 50 °C 1.2: Cooling with ice 2.1: sodium azide; acetic acid / water / 40 °C 3.1: acetic acid / ethanol / Reflux View Scheme |
-
-
633-96-5
orange II

-
-
1386994-22-4
4-(2-nitrobenzylideneamino)naphthalene-1,2-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium dithionite / 40 - 50 °C 2: iron(III) chloride; hydrogenchloride / Cooling with ice 3: sodium azide / water; acetic acid / 2 h / 40 °C 4: acetic acid / ethanol; methanol / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium dithionite / 40 - 50 °C 1.2: Cooling with ice 2.1: sodium azide; acetic acid / water / 40 °C 3.1: acetic acid / ethanol / Reflux View Scheme |
-
-
633-96-5
orange II

-
-
1386994-23-5
4-(4-hydroxy-3-methoxybenzylideneamino)naphthalene-1,2-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium dithionite / 40 - 50 °C 2: iron(III) chloride; hydrogenchloride / Cooling with ice 3: sodium azide / water; acetic acid / 2 h / 40 °C 4: acetic acid / ethanol; methanol / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium dithionite / 40 - 50 °C 1.2: Cooling with ice 2.1: sodium azide; acetic acid / water / 40 °C 3.1: acetic acid / ethanol / Reflux View Scheme |
-
-
633-96-5
orange II

-
-
1386994-24-6
4-(2-hydroxybenzylideneamino)naphthalene-1,2-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium dithionite / 40 - 50 °C 2: iron(III) chloride; hydrogenchloride / Cooling with ice 3: sodium azide / water; acetic acid / 2 h / 40 °C 4: acetic acid / ethanol; methanol / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium dithionite / 40 - 50 °C 1.2: Cooling with ice 2.1: sodium azide; acetic acid / water / 40 °C 3.1: acetic acid / ethanol / Reflux View Scheme |
-
-
633-96-5
orange II

-
-
1386994-25-7
4-((1H-indol-3-yl)methyleneamino)naphthalene-1,2-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium dithionite / 40 - 50 °C 2: iron(III) chloride; hydrogenchloride / Cooling with ice 3: sodium azide / water; acetic acid / 2 h / 40 °C 4: acetic acid / ethanol; methanol / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium dithionite / 40 - 50 °C 1.2: Cooling with ice 2.1: sodium azide; acetic acid / water / 40 °C 3.1: acetic acid / ethanol / Reflux View Scheme |
-
-
633-96-5
orange II

-
-
1386994-26-8
4-((3-phenylallylidene)amino)naphthalene-1,2-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium dithionite / 40 - 50 °C 2: iron(III) chloride; hydrogenchloride / Cooling with ice 3: sodium azide / water; acetic acid / 2 h / 40 °C 4: acetic acid / ethanol; methanol / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium dithionite / 40 - 50 °C 1.2: Cooling with ice 2.1: sodium azide; acetic acid / water / 40 °C 3.1: acetic acid / ethanol / Reflux View Scheme |
-
-
633-96-5
orange II

-
-
1386994-27-9
4-(cyclohexylideneamino)naphthalene-1,2-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium dithionite / 40 - 50 °C 2: iron(III) chloride; hydrogenchloride / Cooling with ice 3: sodium azide / water; acetic acid / 2 h / 40 °C 4: sulfuric acid / ethanol / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium dithionite / 40 - 50 °C 1.2: Cooling with ice 2.1: sodium azide; acetic acid / water / 40 °C 3.1: sulfuric acid / ethanol / Reflux View Scheme |
-
-
633-96-5
orange II

-
-
1386994-28-0
4-(cyclopentylideneamino)naphthalene-1,2-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium dithionite / 40 - 50 °C 2: iron(III) chloride; hydrogenchloride / Cooling with ice 3: sodium azide / water; acetic acid / 2 h / 40 °C 4: sulfuric acid / ethanol / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium dithionite / 40 - 50 °C 1.2: Cooling with ice 2.1: sodium azide; acetic acid / water / 40 °C 3.1: sulfuric acid / ethanol / Reflux View Scheme |
-
-
633-96-5
orange II

-
-
1386994-11-1
4-(benzylideneamino)naphthalene-1,2-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium dithionite / 40 - 50 °C 2: iron(III) chloride; hydrogenchloride / Cooling with ice 3: sodium azide / water; acetic acid / 2 h / 40 °C 4: acetic acid / ethanol; methanol / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium dithionite / 40 - 50 °C 1.2: Cooling with ice 2.1: sodium azide; acetic acid / water / 40 °C 3.1: acetic acid / ethanol / Reflux View Scheme |
-
-
633-96-5
orange II

-
-
1386994-12-2
4-(4-nitrobenzylideneamino)naphthalene-1,2-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium dithionite / 40 - 50 °C 2: iron(III) chloride; hydrogenchloride / Cooling with ice 3: sodium azide / water; acetic acid / 2 h / 40 °C 4: acetic acid / ethanol; methanol / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium dithionite / 40 - 50 °C 1.2: Cooling with ice 2.1: sodium azide; acetic acid / water / 40 °C 3.1: acetic acid / ethanol / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium dithionite / 40 - 50 °C 2: iron(III) chloride; hydrogenchloride / Cooling with ice View Scheme | |
| Stage #1: orange II With sodium dithionite at 40 - 50℃; Stage #2: With hydrogenchloride; iron(III) chloride In water Cooling with ice; |
-
-
633-96-5
orange II

-
-
1386994-13-3
4-(2,4-dichlorobenzylideneamino)naphthalene-1,2-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium dithionite / 40 - 50 °C 2: iron(III) chloride; hydrogenchloride / Cooling with ice 3: sodium azide / water; acetic acid / 2 h / 40 °C 4: acetic acid / ethanol; methanol / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium dithionite / 40 - 50 °C 1.2: Cooling with ice 2.1: sodium azide; acetic acid / water / 40 °C 3.1: acetic acid / ethanol / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: orange II With sodium dithionite at 40 - 50℃; Stage #2: With hydrogenchloride; tin(ll) chloride In water |
Acid Orange 7 Chemical Properties
Product Name: Acid Orange 7 (CAS NO.633-96-5)
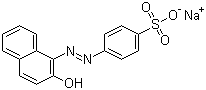
Molecular Formula: C16H11N2NaO4S
Molecular Weight: 350.32g/mol
Mol File: 633-96-5.mol
EINECS: 211-199-0
Appearance: Orange-red powder
Melting Point: 164 °C
Water Solubility: 116 g/L (30 °C)
H-Bond Donor: 1
H-Bond Acceptor: 6
Structure Descriptors of Acid Orange 7 (CAS NO.633-96-5):
IUPAC Name: sodium 4-[(2E)-2-(2-oxonaphthalen-1-ylidene)hydrazinyl]benzenesulfonate
Canonical SMILES: C1=CC=C2C(=C1)C=CC(=O)C2=NNC3=CC=C(C=C3)S(=O)(=O)[O-].[Na+]
Isomeric SMILES: C1=CC=C\2C(=C1)C=CC(=O)/C2=N/NC3=CC=C(C=C3)S(=O)(=O)[O-].[Na+]
InChI: InChI=1S/C16H12N2O4S.Na/c19-15-10-5-11-3-1-2-4-14(11)16(15)18-17-12-6-8-13(9-7-12)23(20,21)22;/h1-10,17H,(H,20,21,22);/q;+1/p-1/b18-16+;
InChIKey: IGQFKZCLTLAWLO-HYNBPGMHSA-M
Product Categories: Organics
Acid Orange 7 Uses
Acid Orange 7 (CAS NO.633-96-5) is one of the most common dyes used for wool, silk, paper, etc. It is also used as indicator and biological colourants.
Acid Orange 7 Toxicity Data With Reference
| 1. | msc-hmn:lyms 12 nmol/L | MUREAV Mutation Research. 249 (1991),265. |
Acid Orange 7 Consensus Reports
Reported in EPA TSCA Inventory.
Acid Orange 7 Safety Profile
The safety information of Acid Orange 7 (CAS NO.633-96-5) is as follows:
Hazard Codes:Xi 
Risk Statements:36/37/38
36/37/38:Irritating to eyes, respiratory system and skin
Safety Statements:26-36-37/39
26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36:Wear suitable protective clothing
37/39:Wear suitable gloves and eye/face protection
WGK Germany:3
RTECS:DB7084000
Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic vapors of NOx and SOx.
Acid Orange 7 Specification
Acid Orange 7 , its CAS NO. is 633-96-5, the synonyms are 4-((2-Hydroxy-1-naphthalenyl)azo)benzenesulfonic acid,
monosodium salt ; Acid orange ii ; Benzenesulfonic acid, 4-((2-hydroxy-1-naphthalenyl)azo)-, monosodium salt ; D & C Orange no. 4 ; Orange II ; 1-p-Sulfophenylazo-2-naphthol, monosodium salt ; 2-Naphthol Orange II ; 4-((2-Hydroxy-1-naphthyl)azo)benzenesulfonic acid, sodium salt ; Acid Leather Orange Extra .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View