-
Name
Allyl Methanethiosulfonate
- EINECS
- CAS No. 14202-77-8
- Density 1.221 g/cm3
- Solubility
- Melting Point NA
- Formula C4H8O2S2
- Boiling Point 271.4 °C at 760 mmHg
- Molecular Weight 152.24
- Flash Point 117.9 °C
- Transport Information
- Appearance Clear colourless liquid
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Methanesulfonicacid, thio-, S-allyl ester (8CI);Methanesulfonothioic acid, S-2-propenyl ester(9CI);S-prop-2-en-1-yl Methanesulfonothioate;
- PSA 67.82000
- LogP 1.94600
Allyl methanethiosulfonate Specification
The Methanesulfonothioicacid, S-2-propen-1-yl ester with CAS registry number of 14202-77-8 is also known as Allyl methanethiosulfonate. The systematic name is S-prop-2-en-1-yl Methanesulfonothioate. It belongs to product categories of MTS and Sulfhydryl Active Reagents; MTS & Sulfhydryl Active Reagents. In addition, the formula is C4H8O2S2 and the molecular weight is 152.24. What's more, this chemical is a clear colourless liquid and it is used to probe the structures of the ACh receptor channel of the GABA receptor channel and of lactose permease.
Physical properties about Methanesulfonothioicacid, S-2-propen-1-yl ester are: (1)ACD/LogP: 1.09; (2)ACD/LogD (pH 5.5): 1.09; (3)ACD/LogD (pH 7.4): 1.09; (4)ACD/BCF (pH 5.5): 3.94; (5)ACD/BCF (pH 7.4): 3.94; (6)ACD/KOC (pH 5.5): 92.94; (7)ACD/KOC (pH 7.4): 92.94; (8)#H bond acceptors: 2 ; (9)#Freely Rotating Bonds: 3; (10)Index of Refraction: 1.508; (11)Molar Refractivity: 37.16 cm3; (12)Molar Volume: 124.6 cm3; (13)Surface Tension: 40.3 dyne/cm; (14)Density: 1.221 g/cm3; (15)Flash Point: 117.9 °C; (16)Enthalpy of Vaporization: 48.91 kJ/mol; (17)Boiling Point: 271.4 °C at 760 mmHg; (18)Vapour Pressure: 0.0108 mmHg at 25 °C.
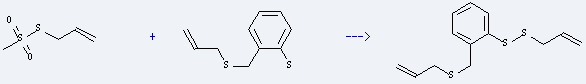
Uses of Methanesulfonothioicacid, S-2-propen-1-yl ester: it is used to produce allyl o-(allylthiomethyl)phenyl disulfide by reaction with allyl (o-mercaptophenyl)methyl sulfide. The reaction occurs with reagent n-butyllithium and solvents hexane, tetrahydrofuran at ambient temperature for 30 minutes. The yield is about 97%.
You can still convert the following datas into molecular structure:
1. SMILES: O=S(=O)(SC\C=C)C
2. InChI: InChI=1/C4H8O2S2/c1-3-4-7-8(2,5)6/h3H,1,4H2,2H3
3. InChIKey: UWKDUBWNZZTSQL-UHFFFAOYAQ
4. Std. InChI: InChI=1S/C4H8O2S2/c1-3-4-7-8(2,5)6/h3H,1,4H2,2H3
5. Std. InChIKey: UWKDUBWNZZTSQL-UHFFFAOYSA-N
Related Products
- ALLYL α-IONONE
- Allyl (2-methylbutoxy)acetate
- Allyl (3-methylbutoxy)acetate
- Allyl 2-(acetylamino)-2-deoxy-3-O-benzyl--D-glucopyranoside
- Allyl 2-acetamido-2-deoxy-beta-D-glucopyranoside
- Allyl 2-chloroethylsulfide
- Allyl 2-ethylbutyrate
- Allyl 2-oxocyclopentanecarboxylate
- Allyl 3,4-epoxy-6-methylcyclohexanecarboxylate
- Allyl 3,5,5-trimethylhexanoate
- 142-03-0
- 14203-19-1
- 142033-88-3
- 142-04-1
- 14204-24-1
- 142044-38-0
- 14205-39-1
- 14205-46-0
- 14205-47-1
- 142055-86-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View