-
Name
3,4-DICHLOROPENTAFLUOROBUTYRIC ACID
- EINECS
- CAS No. 375-07-5
- Article Data9
- CAS DataBase
- Density 1.772 g/cm3
- Solubility
- Melting Point
- Formula C4HCl2F5O2
- Boiling Point 156 °C at 760 mmHg
- Molecular Weight 246.949
- Flash Point 48.1 °C
- Transport Information
- Appearance
- Safety 26-36/37/39-45
- Risk Codes 34
-
Molecular Structure
- Hazard Symbols
- Synonyms Butyricacid, 3,4-dichloro-2,2,3,4,4-pentafluoro- (7CI,8CI);3,4-Dichloro-2,2,3,4,4-pentafluorobutanoic acid;Butanoic acid, 3,4-dichloro-2,2,3,4,4-pentafluoro-;3,4-Dichloropentafluorobutyric acid;
- PSA 37.30000
- LogP 2.44250
Butanoic acid,3,4-dichloro-2,2,3,4,4-pentafluoro- Specification
The Butanoic acid,3,4-dichloro-2,2,3,4,4-pentafluoro-, with the CAS registry number 375-07-5, has the systematic name of 3,4-dichloro-2,2,3,4,4-pentafluorobutanoic acid. It is a kind of organics, and should be stored in the dry and cool environment. And the molecular formula of the chemical is C4HCl2F5O2.
The characteristics of Butanoic acid,3,4-dichloro-2,2,3,4,4-pentafluoro- are as followings: (1)ACD/LogP: 4.64; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.9; (4)ACD/LogD (pH 7.4): 0.89; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1.46; (8)ACD/KOC (pH 7.4): 1.41; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 26.3 Å2; (13)Index of Refraction: 1.383; (14)Molar Refractivity: 32.55 cm3; (15)Molar Volume: 139.3 cm3; (16)Polarizability: 12.9×10-24cm3; (17)Surface Tension: 28.7 dyne/cm; (18)Density: 1.772 g/cm3; (19)Flash Point: 48.1 °C; (20)Enthalpy of Vaporization: 43.32 kJ/mol; (21)Boiling Point: 156 °C at 760 mmHg; (22)Vapour Pressure: 1.56 mmHg at 25°C.
Preparation of Butanoic acid,3,4-dichloro-2,2,3,4,4-pentafluoro-: This chemical can be prepared by 1,2-dichloro-1,1,2,3,3,4,4-heptafluoro-butane. The reaction will need reagent Cl2 and nitrogen oxides. The reaction temperature of 500°C, and the yield is about 72%.

Uses of Butanoic acid,3,4-dichloro-2,2,3,4,4-pentafluoro-: It can be used produce 2,2,3,4,4-pentafluoro-but-3-enoic acid. This reaction will need reagent Zn - dust, and the menstruum dioxane. The reaction time is 4 hours with heating, and the yield is about 73.6%.
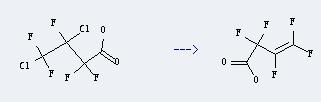
You should be cautious while dealing with this chemical. It may cause burns. Therefore, you had better take the following instructions: Wear suitable protective clothing, gloves and eye/face protection, and if in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice; In case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
Addtionally, the following datas could be converted into the molecular structure:
(1)SMILES: ClC(F)(C(F)(F)C(=O)O)C(Cl)(F)F
(2)InChI: InChI=1/C4HCl2F5O2/c5-3(9,4(6,10)11)2(7,8)1(12)13/h(H,12,13)
(3)InChIKey: WVWCLNDERHVFSB-UHFFFAOYAO
Related Products
- Butanoic acid, 2,2-difluoro-, ethyl ester
- Butanoic acid, 2,3-diamino-
- Butanoic acid, 2-[(6-amino-9H-purin-8-yl)thio]-
- Butanoic acid, 2-amino-4-(methylsulfonyl)-, (2S)-
- Butanoic acid, 2-amino-4-phosphono-, (2R)-
- Butanoic acid, 2-bromo-, (2R)-
- Butanoic acid, 2-bromo-3-oxo-, ethyl ester
- Butanoic acid, 2-isocyanato-3-methyl-, methyl ester, (2S)-
- Butanoic acid, 2-methyl-4-nitro-, (R)- (9CI)
- Butanoic acid, 3-((5-(2-chloro-4-(trifluoromethyl)phenoxy)-2-nitrophenyl)amino)-, methyl ester
- 37509-14-1
- 37511-62-9
- 3751-48-2
- 375-16-6
- 37517-26-3
- 37517-28-5
- 37517-30-9
- 37517-81-0
- 3751-82-4
- 375-19-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View