-
Name
Ethyl 3-methyl-3-phenylglycidate
- EINECS 201-061-8
- CAS No. 77-83-8
- Article Data25
- CAS DataBase
- Density 1.139 g/cm3
- Solubility
- Melting Point
- Formula C12H14O3
- Boiling Point 273.5 °C at 760 mmHg
- Molecular Weight 206.241
- Flash Point 109.371 °C
- Transport Information UN 1993 3/PG 3
- Appearance clear yellow liquid
- Safety 16-26-36/37/39
- Risk Codes 10-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Hydrocinnamicacid, a,b-epoxy-b-methyl-, ethyl ester (7CI,8CI);Oxiranecarboxylic acid, 3-methyl-3-phenyl-,ethyl ester (9CI);3-Methyl-3-phenylglycidic acid ethyl ester;Ethyl methylphenylglycidate;Ethyl a,b-epoxy-b-methylhydrocinnamate;Strawberry aldehyde;
- PSA 38.83000
- LogP 1.86370
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: ethyl dibromoacetate; acetophenone With samarium diiodide In tetrahydrofuran at 20℃; for 2h; Stage #2: With potassium hexamethyldisilazane In tetrahydrofuran; toluene | 90% |

| Conditions | Yield |
|---|---|
| With potassium n-butoxide | 88% |
| With sodium hydride In acetonitrile; mineral oil at 50 - 80℃; optical yield given as %de; | 64% |
| With sodium ethanolate Erhitzen auf 100grad; |

-
-
77-83-8
fraeseol

| Conditions | Yield |
|---|---|
| With potassium carbonate | 80% |
-
-
91767-65-6
2-chloro-3-hydroxy-3-phenyl-butyric acid ethyl ester

-
-
77-83-8
fraeseol

| Conditions | Yield |
|---|---|
| With potassium carbonate | 78% |

| Conditions | Yield |
|---|---|
| methyltrioxorhenium(VII) at 50 - 60℃; for 72h; | 57% |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 86 percent / zinc chloride/silver acetate/bronze-coloured C8K / tetrahydrofuran / 0.33 h / -78 °C 2: 80 percent / K2CO3 View Scheme | |
| Multi-step reaction with 2 steps 1: 90 percent / zinc chloride/silver acetate/bronze-coloured C8K / tetrahydrofuran / 0.67 h / -20 °C 2: 78 percent / K2CO3 View Scheme |
-
-
945-93-7, 13979-22-1, 1504-72-9
ethyl (E)-3-phenylbut-2-enoate

-
-
77-83-8
fraeseol

| Conditions | Yield |
|---|---|
| With 2-Ethylhexanoic acid; C36H40F6MnN6O6S2; dihydrogen peroxide In acetonitrile at 0℃; for 1h; Reagent/catalyst; enantioselective reaction; | 55 %Spectr. |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium hydride / tetrahydrofuran; mineral oil / 0.5 h / 20 °C / Inert atmosphere 1.2: 0 - 20 °C / Inert atmosphere 2.1: C36H40F6MnN6O6S2; 2-Ethylhexanoic acid; dihydrogen peroxide / acetonitrile / 1 h / 0 °C View Scheme |
-
-
77-83-8
fraeseol

| Conditions | Yield |
|---|---|
| With formic acid; triethylamine; Pd0-EnCatTM In ethyl acetate at 23℃; for 24h; | 94% |
-
-
77-83-8
fraeseol

-
-
72655-88-0, 30913-58-7
2-Hydroxy-3-phenyl-3-butensaeureaethylester

| Conditions | Yield |
|---|---|
| With perchloric acid In benzene at 80℃; for 0.5h; Inert atmosphere; | 93% |
| With Nafion-H In dichloromethane Heating; | 70% |

| Conditions | Yield |
|---|---|
| With hydrazinium sulfate at 50℃; for 20h; | 91% |
| Irradiation; |

| Conditions | Yield |
|---|---|
| With hydrazinium sulfate at 50℃; for 48h; | 90% |
-
-
77-83-8
fraeseol

-
-
866457-94-5
rac-(3-methyl-3-phenyloxiran-2-yl)methanol

| Conditions | Yield |
|---|---|
| With C33H29FeMnN2O3P; hydrogen; potassium carbonate at 90℃; under 37503.8 Torr; for 16h; Autoclave; | 71% |

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol at 20℃; for 14h; | 65% |

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol at 45℃; Green chemistry; diastereoselective reaction; | 60% |

| Conditions | Yield |
|---|---|
| With magnesium In N,N,N,N,N,N-hexamethylphosphoric triamide at 60℃; for 48h; | 58% |
-
-
77-83-8
fraeseol

| Conditions | Yield |
|---|---|
| With sodium hydroxide; pig's liver esterase In phosphate buffer at 25℃; for 9h; pH=8; | A 57% B 36% |

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol at 45℃; Green chemistry; diastereoselective reaction; | 47% |

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol at 45℃; Green chemistry; diastereoselective reaction; | 44% |

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol at 45℃; Green chemistry; diastereoselective reaction; | 43% |

| Conditions | Yield |
|---|---|
| Stage #1: fraeseol With sodium azide; sulfuric acid In dimethyl sulfoxide at 20 - 90℃; for 2h; Inert atmosphere; Stage #2: With thionyl chloride; triethylamine In dichloromethane at 20℃; for 24h; Inert atmosphere; Molecular sieve; Cooling with ice; | A 5% B 6% |
-
-
77-83-8
fraeseol

-
-
60727-92-6
2-Phenyl-2-(ethoxycarbonyl)propionaldehyde

| Conditions | Yield |
|---|---|
| With boron trifluoride; benzene |

| Conditions | Yield |
|---|---|
| With ethanol; sodium |
-
-
77-83-8
fraeseol

-
-
34713-70-7
2-Phenylpropanal

| Conditions | Yield |
|---|---|
| With sodium hydroxide Erhitzen des Reaktionsprodukts auf 180grad unter Durchleiten von Wasserdampf; (+-)-2-phenyl-propionaldehyde; |
-
-
77-83-8
fraeseol

| Conditions | Yield |
|---|---|
| With ammonia |

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
77-83-8
fraeseol

-
-
60-29-7
diethyl ether

-
-
58671-17-3
Ethyl-4-ethoxy-3-hydroxy-2-methyl-3-phenylpentanoat

| Conditions | Yield |
|---|---|
| Irradiation; |
-
-
77-83-8
fraeseol

-
-
1074-52-8
ammonium N-phenyldithiocarbamate

-
-
35610-75-4
5-[(methyl)(phenyl)methylidene]-2-thioxo-1,3-thiazolidin-4-one

| Conditions | Yield |
|---|---|
| In ethanol at 35℃; for 720h; |
-
-
77-83-8
fraeseol

-
-
60204-53-7
(4a,6a-dimethyl-tetrahydro-1,4-dioxa-6b-aza-cyclopenta[cd]pentalen-2a-yl)-methanol

-
-
60204-84-4
3-methyl-3-phenyl-oxiranecarboxylic acid 4a,6a-dimethyl-tetrahydro-1,4-dioxa-6b-aza-cyclopenta[cd]pentalen-2a-ylmethyl ester

| Conditions | Yield |
|---|---|
| (i) Na, heptane, (ii) /BRN= 12299/; Multistep reaction; |

| Conditions | Yield |
|---|---|
| In ethanol at 35℃; for 504h; |
-
-
77-83-8
fraeseol

-
-
24744-96-5
ethyl 3-hydroxy-2-methyl-3-phenylpropionate

| Conditions | Yield |
|---|---|
| In diethyl ether Irradiation; |
-
-
77-83-8
fraeseol

-
-
79963-73-8
3-Fluoro-2-hydroxy-3-phenyl-butyric acid ethyl ester

| Conditions | Yield |
|---|---|
| With hydrogen fluoride In pyridine; dichloromethane at 5℃; |
-
-
77-83-8
fraeseol

| Conditions | Yield |
|---|---|
| With sodium azide; sulfuric acid In dimethyl sulfoxide at 90℃; for 2h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |
Ethyl 3-methyl-3-phenylglycidate Consensus Reports
Reported in EPA TSCA Inventory.
Ethyl 3-methyl-3-phenylglycidate Specification
The Ethyl 3-methyl-3-phenylglycidate, with the CAS registry number 77-83-8, is also known as 3-Methyl-3-phenyl glycidic acid ethyl ester. It belongs to the classification code of Mutation Data . Its EINECS registry number is 201-061-8. This chemical's molecular formula is C12H14O3 and molecular weight is 206.24. What's more, both its IUPAC name and systematic name are the same which is called Ethyl 3-methyl-3-phenyloxirane-2-carboxylate. It should be stored in a cool, dry and well-ventilated place. Because of its pleasant taste and aroma, it finds use in the fragrance industry, in artificial flavors, and in cosmetics. Its end applications include perfumes, soaps, beauty care products, detergents, pharmaceuticals, baked goods, candies, ice cream, and others.
Physical properties about Ethyl 3-methyl-3-phenylglycidate are: (1)ACD/LogP: 1.655; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.66; (4)ACD/LogD (pH 7.4): 1.66; (5)ACD/BCF (pH 5.5): 10.66; (6)ACD/BCF (pH 7.4): 10.66; (7)ACD/KOC (pH 5.5): 189.29; (8)ACD/KOC (pH 7.4): 189.29; (9)#H bond acceptors: 3; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 38.83 Å2; (13)Index of Refraction: 1.525; (14)Molar Refractivity: 55.47 cm3; (15)Molar Volume: 181.009 cm3; (16)Polarizability: 21.99×10-24cm3; (17)Surface Tension: 39.792 dyne/cm; (18)Density: 1.139 g/cm3; (19)Flash Point: 109.371 °C; (20)Enthalpy of Vaporization: 51.185 kJ/mol; (21)Boiling Point: 273.5 °C at 760 mmHg; (22)Vapour Pressure: 0.006 mmHg at 25 °C.
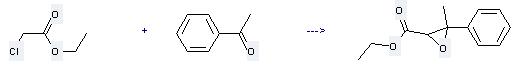
Preparation of Ethyl 3-methyl-3-phenylglycidate: this chemical can be prepared by chloroacetic acid ethyl ester with 1-phenyl-ethanone. This reaction needs reagent sodium ethoxide and solvent diethyl ether at ambient temperature. The reaction time is 1 hour.
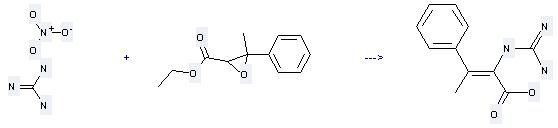
Uses of Ethyl 3-methyl-3-phenylglycidate: it is used to produce other chemicals. For example, it can react with guanidine; nitrate to get 2-guanidino-3-phenylbutanoic acid. The reaction occurs with reagent sodium ethoxide and solvent ethanol at temperature of 25 °C. The reaction time is 14 hours. The yield is 65 %.
When you are dealing with this chemical, you should be very careful. This chemical may cause inflammation to the skin or other mucous membranes and it is flammable. It is irritating to eyes, respiratory system and skin. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. In addition, you should keep away from sources of ignition. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: O=C(OCC)C2OC2(c1ccccc1)C
(2) InChI: InChI=1S/C12H14O3/c1-3-14-11(13)10-12(2,15-10)9-7-5-4-6-8-9/h4-8,10H,3H2,1-2H3
(3) InChIKey: LQKRYVGRPXFFAV-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | oral | 4050mg/kg (4050mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: OTHER CHANGES | Food and Cosmetics Toxicology. Vol. 2, Pg. 327, 1964. |
| rat | LD50 | oral | 5470mg/kg (5470mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE SKIN AND APPENDAGES (SKIN): HAIR: OTHER | Food and Cosmetics Toxicology. Vol. 2, Pg. 327, 1964. |
Related Products
- Ethyl (13-cis)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
- ethyl (1R,2R)-1-phenyl-2-(trideuteriomethylamino)cyclohex-3-ene-1-carboxylate,hydrochloride
- Ethyl (1S,2R)-2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate hydrochloride
- Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate
- Ethyl (2-amino-4-hydroxy-6-methyl-5-pyrimidinyl)acetate
- Ethyl (2-bromopropionamido)acetate
- Ethyl (2-cyanoimino-5,6-dichloro-1,2,3,4-tetrahydroquinazolin-3-yl)acetate
- ETHYL (2E,4Z)-DECADIENOATE
- Ethyl (2-hydroxyethyl)dimethyl-ammonium benzilate chloride
- Ethyl (2-mercaptoethyl) carbamate S-ester with O,O-dimethyl phosphorodithioate
- 7783-82-6
- 7783-83-7
- 7783-85-9
- 7783-86-0
- 7783-89-3
- 7783-90-6
- 7783-92-8
- 7783-93-9
- 7783-95-1
- 7783-96-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View