-
Name
Isopropyl palmitate
- EINECS 205-571-1
- CAS No. 142-91-6
- Article Data22
- CAS DataBase
- Density 0.862 g/cm3
- Solubility Not miscible or difficult to mix with water.
- Melting Point 11-13 °C(lit.)
- Formula C19H38O2
- Boiling Point 340.7 °C at 760 mmHg
- Molecular Weight 298.51
- Flash Point 162.2 °C
- Transport Information
- Appearance Clear liquid
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Isopal;Isopalm;Isopropyl hexadecanoate;Propal;Palmiticacid, isopropyl ester (6CI,7CI,8CI);1-Methylethyl hexadecanoate;
- PSA 26.30000
- LogP 6.41930
Synthetic route

| Conditions | Yield |
|---|---|
| With perchloric acid at 100℃; for 4h; Esterification; | 99.74% |
| With alumina methanesulfonic acid at 120℃; for 0.333333h; Microwave irradiation; | 97% |
| With toluene-4-sulfonic acid at 60℃; for 72h; | 89.3% |
-
-
57-10-3
1-hexadecylcarboxylic acid

-
-
67-63-0
isopropyl alcohol

-
A
-
142-91-6
palmitic acid isopropyl ester

-
B
-
7732-18-5
water

| Conditions | Yield |
|---|---|
| at 140℃; under 3825.38 Torr; | A 64% B 0.06% |
| at 98℃; under 759.826 Torr; | A 14.04% B 0.83% |


| Conditions | Yield |
|---|---|
| With pyridine | |
| In pyridine | |
| at 20℃; for 2h; | |
| With triethylamine In dichloromethane at 0 - 4℃; for 2h; | 0.9% |
-
-
65266-83-3
Hexadecanoic acid 2-(toluene-4-sulfonyloxy)-1-(toluene-4-sulfonyloxymethyl)-ethyl ester

-
-
142-91-6
palmitic acid isopropyl ester

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate |
-
-
17364-07-7, 22147-34-8, 112-11-8
isopropyl oleate

-
-
112-39-0
hexadecanoic acid methyl ester

-
A
-
112-62-9
Methyl oleate

-
B
-
142-91-6
palmitic acid isopropyl ester

| Conditions | Yield |
|---|---|
| With sodium methylate In hexane at 60℃; for 0.0166667h; Product distribution; |

-
-
23470-00-0
2-palmitoylglycerol

-
-
142-91-6
palmitic acid isopropyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Py 2: NaBH4 View Scheme |
-
-
56599-87-2
2-O-palmitoyl-1,3-O-benzylideneglycerol

-
-
142-91-6
palmitic acid isopropyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: H3BO3, B(OEt)3 2: Py 3: NaBH4 View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: iodine / acetonitrile / 0.17 h / Inert atmosphere 2.1: zinc trifluoromethanesulfonate / acetonitrile / 0.5 h / 60 °C / Inert atmosphere 2.2: 5 h / 60 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: C34H46O2P(1+)*I(1-) With zinc trifluoromethanesulfonate In acetonitrile at 60℃; for 0.5h; Inert atmosphere; Stage #2: C27H22O3P(1+)*I(1-) In acetonitrile at 60℃; for 5h; Inert atmosphere; | 45 mg |

| Conditions | Yield |
|---|---|
| With lithium cyclohexylisopropylamide In tetrahydrofuran at -60℃; | 100% |

| Conditions | Yield |
|---|---|
| With sodium ethanolate In methanol; ethanol at 60℃; for 4h; Inert atmosphere; | 89% |
-
-
554-62-1
D-ribo-phytosphingosine

-
-
142-91-6
palmitic acid isopropyl ester

-
-
111149-09-8
N-palmitoyl-D-ribo-phytosphingosine

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol at 55℃; | 82.9% |
-
-
142-91-6
palmitic acid isopropyl ester

-
-
1115-70-4
metformin hydrochloride

-
-
66709-59-9
N2,N2-dimethyl-6-pentadecyl-1,3,5-triazine-2,4-diamine

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol for 2h; Reflux; | 64% |
-
-
142-91-6
palmitic acid isopropyl ester

-
-
55-57-2
1-phenylbiguanide monohydrochloride

-
-
22305-30-2
6-pentadecyl-N2-phenyl-1,3,5-triazine-2,4-diamine

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol for 2h; Reflux; | 42% |
-
-
142-91-6
palmitic acid isopropyl ester

| Conditions | Yield |
|---|---|
| With cells of Rhodococcus sp. strain KSM-MT66 In water at 26℃; pH=7; Dehydrogenation; | |
| With glutamic acid sodium salt; resting cells of a mutant; Rhodococcus sp. strain KSM-MT66 In phosphate buffer pH=7.0; Product distribution; Dehydrogenation; Microbiological reaction; |
-
-
142-91-6
palmitic acid isopropyl ester

-
-
144664-37-9
(E)-2-((R)-1-Hydroxy-prop-2-ynyl)-hexadec-2-enoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 100 percent / LiN(i-Pr)C6H11 / tetrahydrofuran / -60 °C 2: 1.) BrMgN(i-Pr)2 / 1.) ether, 2.) ether, -40 deg C 3: NaHCO3 / benzene / 1 h / 60 °C 4: aq.KOH / methanol; diethyl ether / Ambient temperature View Scheme |
-
-
142-91-6
palmitic acid isopropyl ester

-
-
144582-64-9
(E)-2-((R)-1-Hydroxy-prop-2-ynyl)-hexadec-2-enoic acid isopropyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 100 percent / LiN(i-Pr)C6H11 / tetrahydrofuran / -60 °C 2: 1.) BrMgN(i-Pr)2 / 1.) ether, 2.) ether, -40 deg C 3: NaHCO3 / benzene / 1 h / 60 °C View Scheme |
-
-
142-91-6
palmitic acid isopropyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 100 percent / LiN(i-Pr)C6H11 / tetrahydrofuran / -60 °C 2: 1.) BrMgN(i-Pr)2 / 1.) ether, 2.) ether, -40 deg C 3: NaHCO3 / benzene / 1 h / 60 °C 4: aq.KOH / methanol; diethyl ether / Ambient temperature 5: Ag2CO3 / benzene / 80 °C View Scheme |
-
-
142-91-6
palmitic acid isopropyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 100 percent / LiN(i-Pr)C6H11 / tetrahydrofuran / -60 °C 2: 1.) BrMgN(i-Pr)2 / 1.) ether, 2.) ether, -40 deg C View Scheme |
Isopropyl palmitate Consensus Reports
Isopropyl palmitate Specification
The Isopropyl palmitate with CAS registry number of 142-91-6 is also called Hexadecanoicacid,1-methylethyl ester. Its EINECS registry number is 205-571-1. The IUPAC name is propan-2-yl hexadecanoate. In addition, the molecular formula is C19H38O2 and the molecular weight is 298.50. It belongs to the classes of Hair Care and Skin Care.
Physical properties about this chemical are: (1)ACD/LogP: 8.50; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 8.49; (4)ACD/LogD (pH 7.4): 8.49; (5)ACD/BCF (pH 5.5): 1000000; (6)ACD/BCF (pH 7.4): 1000000; (7)ACD/KOC (pH 5.5): 995595.94; (8)ACD/KOC (pH 7.4): 995595.94; (9)#H bond acceptors: 2; (10)#Freely Rotating Bonds: 16; (11)Polar Surface Area: 26.3 Å2; (12)Index of Refraction: 1.443; (13)Molar Refractivity: 91.8 cm3; (14)Molar Volume: 346 cm3; (15)Polarizability: 36.39 ×10-24cm3; (16)Surface Tension: 30 dyne/cm; (17)Density: 0.862 g/cm3; (18)Flash Point: 162.2 °C; (19)Enthalpy of Vaporization: 58.43 kJ/mol; (20)Boiling Point: 340.7 °C at 760 mmHg; (21)Vapour Pressure: 8.44E-05 mmHg at 25°C.
Preparation of Isopropyl palmitate: it can be prepared by hexadecanoic acid and isopropanol. Add hexadecanoic acid and isopropanol into the reactor at first. Then add the amount of catalyst sulfuric acid into the mixture and reflux for 10 hours with stirring. After the reaction, steam out the excess isopropyl alcohol and water. Via a series of cooling, neutralization by 5% Na2CO3 aqueous solution, separation and decompression dehydration you can get the desired product.
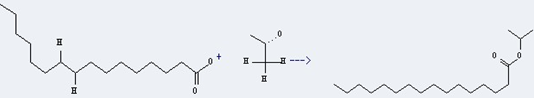
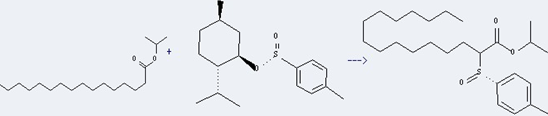
Uses of Isopropyl palmitate: it can be used as emollients, carrier of the oils and fats, solvent and flavor solubilizer. In addition, it can react with (S)-menthyl-p-toluenesulfinate to get isopropyl (R&Se). This reaction will need reagent LiN(i-Pr)C6H11 and solvent tetrahydrofuran. The yield is about 100% at reaction temperature of -60 °C.
When you are using this chemical, please be cautious about it as the following:
It is irritating to eyes, respiratory system and skin. When you are using it, wear suitable protective clothing. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OC(C)C)CCCCCCCCCCCCCCC
(2)InChI: InChI=1/C19H38O2/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17-19(20)21-18(2)3/h18H,4-17H2,1-3H3
(3)InChIKey: XUGNVMKQXJXZCD-UHFFFAOYAX
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 100mg/kg (100mg/kg) | National Technical Information Service. Vol. AD277-689, | |
| rabbit | LD50 | skin | > 5gm/kg (5000mg/kg) | Food and Chemical Toxicology. Vol. 20, Pg. 727, 1982. | |
| rat | LD50 | oral | > 5gm/kg (5000mg/kg) | Food and Chemical Toxicology. Vol. 20, Pg. 727, 1982. |
Related Products
- ISOPROPYL γ-FLUOROBUTYRATE
- Isopropyl (2S)-2-hydroxypropanoate
- Isopropyl 2-(3-nitrobenzylidene)acetoacetate
- Isopropyl 2,4-dichlorophenoxyacetate
- Isopropyl 2-bromo-2-methylpropanoate
- Isopropyl 2-cyanoacetate
- Isopropyl 2-methylbutanoate
- Isopropyl 3-amino-4-methylbenzoate
- Isopropyl 3-aminocrotonate
- Isopropyl 4-hydroxypiperidine-1-carboxylate
- 14292-23-0
- 14292-26-3
- 14292-27-4
- 142925-36-8
- 142-92-7
- 142929-10-0
- 142929-49-5
- 142931-47-3
- 14293-44-8
- 14293-50-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View