Luyunjia Chemistry Xiamen Limited
We are constantly upgrading every aspect of our quality systems in terms of staff training, documentation and analytical capabilities to Overview Quick Details CAS No.: 112529-15-4 MF: C19H21ClN2O3S Place of Origin: Shandong, China (Mainland)
Cas:112529-15-4
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryDayang Chem (Hangzhou) Co.,Ltd.
Dayangchem's R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. DayangChem can provide different quantities
Cas:112529-15-4
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryHenan Allgreen Chemical Co.,Ltd
high quality Appearance:Solid Storage:Sealed, dry, microtherm , avoid light and smell. Package:According to the demand of customer Application:Intermediates Transportation:by air or by sea Port:shanghai
Cas:112529-15-4
Min.Order:1 Kilogram
Negotiable
Type:Manufacturers
inquiryXi'an Xszo Chem Co., Ltd.
1. Factory price and high quality must be guaranteed, base on 8 years of production and R&D experience2. Free samples will be provided,ensure specifications and quality are right for customer3. Customers will receive the most professional technical s
Cas:112529-15-4
Min.Order:1 Gram
FOB Price: $0.1
Type:Manufacturers
inquiryChemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Cas:112529-15-4
Min.Order:5 Kiloliter
FOB Price: $1.2 / 5.0
Type:Manufacturers
inquiryKono Chem Co.,Ltd
Xi'an Kono Chem Co., Ltd., founded in 2014, is a holding enterprise of Hongkong Pioneer Biotech Group. It is an export-oriented manufacturing enterprise supported by the Ministry of Commerce. Kono Chem is located in Xi'an, Shaanxi Pr
Cas:112529-15-4
Min.Order:1 Kilogram
FOB Price: $600.0 / 620.0
Type:Other
inquiryLIDE PHARMACEUTICALS LIMITED
LIDE PHARMACEUTICALS LIMITED is a professional chemicals and APIs leading manufacturer in China. Our core business line covers APIs, Intermediates, Herb extract, etc.
Cas:112529-15-4
Min.Order:1 Kilogram
FOB Price: $0.9 / 1.0
Type:Lab/Research institutions
inquiryHenan Tianfu Chemical Co., Ltd.
Product Name: Pioglitazone hydrochloride Synonyms: (+-5-[[4-[2-(5-ETHYL-2-PYRIDINYL)ETHOXY] PHENYL]METHYL]-2 4-] THIAZOLIDINEDIONE H YDROCHLORIDE(PIOGLITAZONE HCL);Pioglitazone hydrochloride, 99%, receptor-gamma (PPARgamma) agonist;Pioglitazone hy
Cas:112529-15-4
Min.Order:1 Kilogram
FOB Price: $230.0
Type:Lab/Research institutions
inquiryHebei Nengqian Chemical Import and Export Co., LTD
With our good experience, we offer detailed technical support and advice to assist customers. We communicate closely with customers to establish their quality requirements. Consistent Quality Our plant has strict quality control in each manufacturin
Cas:112529-15-4
Min.Order:1 Kilogram
FOB Price: $3.0 / 10.0
Type:Trading Company
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:112529-15-4
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by email in time prod
Cas:112529-15-4
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryWuhan Han Sheng New Material Technology Co.,Ltd
Our Advantage: high quality with competitive price High quality standard: BP/USP/EP Enterprise standard All purity customized Fast and safe delivery We have reliable forwarder who can help us deliver our goods more fast and safe. We
Henan Sinotech Import&Export Corporation
Name Pioglitazone hydrochloride CAS 112529-15-4 Purity 99%min Appearance white powders Appearance:White powder Storage:Store in cool and dry place,
Cas:112529-15-4
Min.Order:1 Metric Ton
FOB Price: $1.0 / 2.0
Type:Other
inquiryShijiazhuang Sdyano Fine Chemical Co., Ltd
1. Best prices with satisfied quality; 2. It's clients' right to choose the package's Courier (EMS, DHL, FedEx, UPS); 3.It's clients' right to choose the packing way for his products from many recent effective packing ways;
Cas:112529-15-4
Min.Order:0 Metric Ton
Negotiable
Type:Trading Company
inquiryHubei Langyou International Trading Co., Ltd
Our Adwantage: 1.We have stock so we can delivery quickly at the very day when receive the payment. 2.Best price, first class service, high successful delivery rate. A discount would be given when you make a large order. 3.High quality gua
Cas:112529-15-4
Min.Order:10 Gram
Negotiable
Type:Other
inquiryHANWAYS CHEMPHARM CO.,LIMITED
1.Prompt Goods; 2.Flexible payment terms; 3.Documents & Certificate support. Name: Pioglitazone hydrochloride Molecular Structure: Formula: C19H21ClN2O3S Molecular Weight :392.9 Synonyms: 5-[4-[2-(N-Methyl-N-(2-pyridyl)amino)ethoxy]benzyl
Cas:112529-15-4
Min.Order:1 Kilogram
Negotiable
Type:Trading Company
inquiryShanghai Upbio Tech Co.,Ltd
1,In No Less five years exporting experience. 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Onchem specialized in APIs, chemical intermediate
Cas:112529-15-4
Min.Order:25 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryXi'an Faithful Biotech Co., Ltd.
We are the manufacturers and suppliers of API in China, and warehouse in Germany and USA of California, which can quickly and safely deliver to your address 1.High quality and competitive price. 2.Free sample for your evaluation. 3.Promptly delivery
Cas:112529-15-4
Min.Order:10 Gram
FOB Price: $3.5
Type:Trading Company
inquiryBaoji Guokang Healthchem co.,ltd
Our company has been in existence for 10 years since its establishment. We have our own unique team. The company integrates independent research and development, production and sales. We have established famous brands at home and abroad. At prese
Cas:112529-15-4
Min.Order:1 Gram
FOB Price: $7.2 / 8.4
Type:Trading Company
inquiryQingdao Beluga Import and Export Co., LTD
Pioglitazone hydrochloride CAS:112529-15-4 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organ
Cas:112529-15-4
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryChangchun Artel lmport and Export trade company
Product Detail Minimum Order Qty. 10 Gram Supply Ability 500 Kilograms/Month Storage store in cool, dry, ventilated place 20℃ Delivery Time 3 business days after payment Payment Term TT,western union,Paypal,
Cas:112529-15-4
Min.Order:20 Metric Ton
Negotiable
Type:Trading Company
inquiryWuhan Wonda Pharm Limited
1.High Quality: Quality is life. Quality is the most important element for all goods. We have a lab doing research in Wuhan China and produce sarms in bulk quantity. We have 8 years experience making all kinds of sarms. And all our old customers
Cas:112529-15-4
Min.Order:100 Gram
Negotiable
Type:Lab/Research institutions
inquiryHubei Jiutian Bio-medical Technology Co., Ltd
1,we produce and sell good chemicals around the world. 2,our success rate is about 95%. this means, if customer order is accepted, the probability that the customer will obtain the ordered substances, is 95%. 3,our staff consists of highly qualifie
Cas:112529-15-4
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHubei CuiRan Biotechnology Co., Ltd
Hubei CuiRan Biotechnology Co., Ltd is a leading company in the research, development, manufacture and marketing of High Quality Phytochemicals and Extracts(especially Active Ingredients from Traditional Chinese Medicine,Traditional Chinese Medicine)
Cas:112529-15-4
Min.Order:10 Milligram
Negotiable
Type:Lab/Research institutions
inquiryHangzhou JINLAN Pharm-Drugs Technology Co., Ltd
We can provide GMP validation service that complies with SFDA, FDA, WHO and EU EMPA.Excellent registration team could help us easlily to register our products in different countries.If you and your customer are interested in some products or need C
Cas:112529-15-4
Min.Order:1 Gram
Negotiable
Type:Other
inquiryHebei Mojin Biotechnology Co.,Ltd
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional chemical raw materials and chemical reagents research and development production enterprises. We have several production line,So we can control the lowest price. We also have several
Cas:112529-15-4
Min.Order:25 Gram
FOB Price: $90.0 / 100.0
Type:Trading Company
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:112529-15-4
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryTriumph International Development Limilted
Appearance:white or light yellow crystalline powder Storage:Store in a cool,dry place and keep away from direct strong light Package:As customer request Application:Used for research and industrial manufacture. Transportation:By
Afine Chemicals Limited
Our Services 1. New Molecules R&D 2. Own test center HPLC NMR GC LC-MS 3. API and Intermediates from China reputed manufacturers 4. Documents support COA MOA MSDS DMF open part Our advantages 1. Government awarded company. Top 100 enter
Cas:112529-15-4
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryWuhan Zenuo Biological Medicine Technology Co Ltd
Product Name: Pioglitazone hydrochloride Synonyms: Pioglitazone hydrochloride, 99%, receptor-gamma (PPARgamma) agonist;Pioglitazone hydrochloride, >=99%;thiazolidinedione hydrochloride;5-[4-[2-(5-Ethyl-2-pyridyl)ethoxy]benzyl]thiazolidine-2,4
Cas:112529-15-4
Min.Order:100 Gram
Negotiable
Type:Lab/Research institutions
inquirySynthetic route
-
-
105355-27-9
Pioglitazone

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol at 10 - 65℃; for 4.25h; Product distribution / selectivity; | 95% |
| With hydrogenchloride In ethanol; water at 10 - 65℃; for 4.25h; Product distribution / selectivity; | 90% |
| With hydrogenchloride In methanol; water at 20℃; for 1h; | 90.5% |

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methylene]-thiazolidine-2,4-dione hydrogen chloride With hydrogen; palladium 10% on activated carbon In methanol; water at 50 - 60℃; under 3750.38 - 4500.45 Torr; for 15h; Stage #2: With hydrogenchloride In water at 0 - 5℃; for 3h; | 84% |
-
-
5223-06-3
5-ethyl-2-(2-hydroxyethyl)pyridine

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 63 percent / NaH / dimethylformamide 2: H2 / 10percent Pd-C / methanol / 760 Torr / Ambient temperature 3: 1.)47percent HBr, NaNO2, 2.)Cu2O / 1.)MeOH, H2O, 5 deg C, 20 min., 2.)40 deg C 4: 53 percent / NaOAc / ethanol / Heating 5: 2N HCl / 6 h / Heating 6: 12.2 g / 11percent ethanolic HCl / 1 h / Ambient temperature View Scheme | |
| Multi-step reaction with 10 steps 1: dihydrogen peroxide / acetic acid / 14 h / 100 °C 2: 0.5 h / 120 °C 3: sodium hydroxide / water / 2 h / 40 °C 4: thionyl chloride; triethylamine / dichloromethane / 1.25 h / 0 - 5 °C 5: N,N-dimethyl-formamide / 14 h / 80 °C 6: pyrrolidine / methanol / 2 h / 50 - 55 °C / Large scale 7: sodium tetrahydroborate; butane-2,3-dione dioxime; cobalt(II) chloride hexahydrate / water; N,N-dimethyl-formamide / 4 h / 65 - 85 °C / Large scale 8: triethylamine / dichloromethane / 2 h / 0 °C 9: acetic acid; sodium tetrahydroborate / 15 h / 40 - 45 °C 10: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 10 steps 1: dihydrogen peroxide / acetic acid / 14 h / 100 °C 2: 0.5 h / 120 °C 3: sodium hydroxide / water / 2 h / 40 °C 4: thionyl chloride; triethylamine / dichloromethane / 1.25 h / 0 - 5 °C 5: N,N-dimethyl-formamide / 14 h / 80 °C 6: pyrrolidine / methanol / 2 h / 50 - 55 °C / Large scale 7: sodium tetrahydroborate; butane-2,3-dione dioxime; cobalt(II) chloride hexahydrate / water; N,N-dimethyl-formamide / 4 h / 65 - 85 °C / Large scale 8: triethylamine 9: acetic acid; sodium tetrahydroborate / 15 h / 40 - 45 °C 10: hydrogenchloride / water; ethanol View Scheme |
-
-
85583-40-0
4-[2-(5-ethylpyridin-2-yl)ethoxy]aniline

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 1.)47percent HBr, NaNO2, 2.)Cu2O / 1.)MeOH, H2O, 5 deg C, 20 min., 2.)40 deg C 2: 53 percent / NaOAc / ethanol / Heating 3: 2N HCl / 6 h / Heating 4: 12.2 g / 11percent ethanolic HCl / 1 h / Ambient temperature View Scheme |
-
-
85583-54-6
4-(2-(5-ethyl-2-pyridyl)ethoxy)nitrobenzene

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: H2 / 10percent Pd-C / methanol / 760 Torr / Ambient temperature 2: 1.)47percent HBr, NaNO2, 2.)Cu2O / 1.)MeOH, H2O, 5 deg C, 20 min., 2.)40 deg C 3: 53 percent / NaOAc / ethanol / Heating 4: 2N HCl / 6 h / Heating 5: 12.2 g / 11percent ethanolic HCl / 1 h / Ambient temperature View Scheme |
-
-
105355-25-7
methyl 2-bromo-3-{4-[2-(5-ethyl-pyridin-2-yl)ethoxy]phenyl}propionate

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 53 percent / NaOAc / ethanol / Heating 2: 2N HCl / 6 h / Heating 3: 12.2 g / 11percent ethanolic HCl / 1 h / Ambient temperature View Scheme |
-
-
105355-26-8
5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2-imino-4-thiazolidinone

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 2N HCl / 6 h / Heating 2: 12.2 g / 11percent ethanolic HCl / 1 h / Ambient temperature View Scheme | |
| With hydrogenchloride; water for 15h; Product distribution / selectivity; Heating / reflux; | |
| Stage #1: 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2-imino-4-thiazolidinone With methanol; oxalic acid In dichloromethane at 20℃; for 0.5h; Stage #2: With hydrogenchloride; water for 12h; Product distribution / selectivity; Heating / reflux; |
-
-
350-46-9
4-Fluoronitrobenzene

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 63 percent / NaH / dimethylformamide 2: H2 / 10percent Pd-C / methanol / 760 Torr / Ambient temperature 3: 1.)47percent HBr, NaNO2, 2.)Cu2O / 1.)MeOH, H2O, 5 deg C, 20 min., 2.)40 deg C 4: 53 percent / NaOAc / ethanol / Heating 5: 2N HCl / 6 h / Heating 6: 12.2 g / 11percent ethanolic HCl / 1 h / Ambient temperature View Scheme |
-
-
674798-32-4
2-bromo-3-{4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl}propionic acid

-
-
17356-08-0
thiourea

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromo-3-{4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl}propionic acid; thiourea With sodium acetate In ethanol for 3h; Heating / reflux; Stage #2: With hydrogenchloride In ethanol; water for 18h; Heating / reflux; Stage #3: With ammonia In ethanol; water at 20℃; |
-
-
1034515-30-4
5-{4-[2-(5-ethyl-pyridin-2-yl)ethoxy]-benzyl}-2-morpholin-4-yl-thiazol-4-one

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water; isopropyl alcohol for 8h; Product distribution / selectivity; Heating / reflux; |
-
-
1034515-42-8
N-(5-{4-[2-(5-ethyl-pyridin-2-yl)-ethoxy]-benzyl}-4-oxo-4,5-dihydrothiazol-2-yl)-methanesulfonamide

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water for 3h; Product distribution / selectivity; Heating / reflux; |
-
-
1034515-24-6
N-(5-{4-[2-(5-ethyl-pyridin-2-yl)ethoxy]benzyl}-4-oxo-4,5-dihydrothiazol-2-yl)-4-methylbenzenesulfonamide

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: N-(5-{4-[2-(5-ethyl-pyridin-2-yl)ethoxy]benzyl}-4-oxo-4,5-dihydrothiazol-2-yl)-4-methylbenzenesulfonamide With hydrogenchloride; water for 8h; Heating / reflux; Stage #2: With hydrogenchloride In ethyl acetate at 25 - 30℃; for 2h; Product distribution / selectivity; |
-
-
144809-28-9
5-{4-[2-(5-ethylpyridn-2-yl)ethoxy]benzylidene}-2,4-thiazolidene dione

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: 5-{4-[2-(5-ethylpyridn-2-yl)ethoxy]benzylidene}-2,4-thiazolidene dione With sodium tetrahydroborate; cobalt(II) nitrate hexahydrate; butane-2,3-dione dioxime; sodium hydroxide In water; N,N-dimethyl-formamide at 35℃; for 6h; Large scale reaction; Stage #2: With acetic acid In water; N,N-dimethyl-formamide at 25℃; for 0.75h; pH=6.5 - 7; Large scale reaction; Stage #3: With hydrogenchloride In water; isopropyl alcohol at 25 - 80℃; Large scale reaction; | 49.6 kg |
| Multi-step reaction with 2 steps 1: palladium 10% on activated carbon; hydrogen / methanol / 16 h / 3102.97 Torr 2: hydrogenchloride / water; ethanol View Scheme |
-
-
646519-90-6
5-{4-[2-(5-ethylpyridin-2-yl)-2-mesylethoxy]benzylidene}-2,4-thiazolidene dione

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium tetrahydroborate; butane-2,3-dione dioxime; cobalt(II) chloride hexahydrate / water; N,N-dimethyl-formamide / 3 h / 60 - 65 °C 2: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 3 steps 1: palladium 10% on activated carbon; hydrogen / methanol / 14 h / 3102.97 Torr 2: acetic acid; sodium tetrahydroborate / 15 h / 40 - 45 °C 3: hydrogenchloride / water; ethanol View Scheme |

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: acetic acid; sodium tetrahydroborate / 15 h / 40 - 45 °C 2: hydrogenchloride / water; ethanol View Scheme |
-
-
646519-92-8
5-{4-[2-(5-ethylpyridin-2-yl)-2-tosylethoxy]benzylidene}-2,4-thiazolidene dione

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: palladium 10% on activated carbon; hydrogen / water; methanol / 14 h / 3102.97 Torr 2: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 2 steps 1: sodium tetrahydroborate; butane-2,3-dione dioxime; cobalt(II) chloride hexahydrate / water; N,N-dimethyl-formamide / 3 h / 60 - 65 °C 2: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 3 steps 1: palladium 10% on activated carbon; hydrogen / water; methanol / 14 h / 3102.97 Torr 2: acetic acid; sodium tetrahydroborate / 15 h / 40 - 45 °C 3: hydrogenchloride / water; ethanol View Scheme |

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: acetic acid; sodium tetrahydroborate / 15 h / 40 - 45 °C 2: hydrogenchloride / water; ethanol View Scheme |
-
-
646519-94-0
5-{4-[2-chloro-2-(5-ethylpyridn-2-yl)ethoxy]benzylidene}-2,4-thiazolidene dione

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogen / tetrahydrofuran / 12 h 2: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 2 steps 1: sodium tetrahydroborate; butane-2,3-dione dioxime; cobalt(II) chloride hexahydrate / water; N,N-dimethyl-formamide / 4 h / 65 - 70 °C 2: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 3 steps 1: zinc; acetic acid / methanol / 15 h / 25 - 30 °C 2: palladium 10% on activated carbon; hydrogen / methanol / 16 h / 3102.97 Torr 3: hydrogenchloride / water; ethanol View Scheme |
-
-
90643-32-6
2-(5-ethyl-1-oxypyridin-2-yl)ethanol

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 0.5 h / 120 °C 2: sodium hydroxide / water / 2 h / 40 °C 3: thionyl chloride; triethylamine / dichloromethane / 1.25 h / 0 - 5 °C 4: N,N-dimethyl-formamide / 14 h / 80 °C 5: pyrrolidine / methanol / 2 h / 50 - 55 °C / Large scale 6: sodium tetrahydroborate; butane-2,3-dione dioxime; cobalt(II) chloride hexahydrate / water; N,N-dimethyl-formamide / 4 h / 65 - 85 °C / Large scale 7: thionyl chloride / chloroform / 2 h / Reflux 8: hydrogenchloride 9: zinc; propionic acid / ethanol / 12 h 10: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 10 steps 1: 0.5 h / 120 °C 2: sodium hydroxide / water / 2 h / 40 °C 3: thionyl chloride; triethylamine / dichloromethane / 1.25 h / 0 - 5 °C 4: N,N-dimethyl-formamide / 14 h / 80 °C 5: thionyl chloride / chloroform / 1 h / Reflux 6: acetic acid; piperidine / toluene / Heating 7: hydrogen / tetrahydrofuran / 12 h 8: hydrogenchloride 9: zinc; propionic acid / ethanol / 12 h 10: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 10 steps 1: 0.5 h / 120 °C 2: sodium hydroxide / water / 2 h / 40 °C 3: thionyl chloride; triethylamine / dichloromethane / 1.25 h / 0 - 5 °C 4: N,N-dimethyl-formamide / 14 h / 80 °C 5: pyrrolidine / methanol / 2 h / 50 - 55 °C / Large scale 6: thionyl chloride / chloroform / 3 h / Reflux 7: hydrogen / tetrahydrofuran / 12 h 8: hydrogenchloride 9: zinc; propionic acid / ethanol / 12 h 10: hydrogenchloride / water; ethanol View Scheme |

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: thionyl chloride; triethylamine / dichloromethane / 1.25 h / 0 - 5 °C 2: N,N-dimethyl-formamide / 14 h / 80 °C 3: thionyl chloride / chloroform / 1 h / Reflux 4: acetic acid; piperidine / toluene / Heating 5: hydrogen / tetrahydrofuran / 12 h 6: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 6 steps 1: thionyl chloride; triethylamine / dichloromethane / 1.25 h / 0 - 5 °C 2: N,N-dimethyl-formamide / 14 h / 80 °C 3: pyrrolidine / methanol / 2 h / 50 - 55 °C / Large scale 4: thionyl chloride / chloroform / 3 h / Reflux 5: hydrogen / tetrahydrofuran / 12 h 6: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 6 steps 1: thionyl chloride; triethylamine / dichloromethane / 1.25 h / 0 - 5 °C 2: N,N-dimethyl-formamide / 14 h / 80 °C 3: pyrrolidine / methanol / 2 h / 50 - 55 °C / Large scale 4: triethylamine / dichloromethane / 2 h / 0 °C 5: palladium 10% on activated carbon; hydrogen / water; methanol / 14 h / 3102.97 Torr 6: hydrogenchloride / water; ethanol View Scheme |

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: N,N-dimethyl-formamide / 14 h / 80 °C 2: thionyl chloride / chloroform / 1 h / Reflux 3: acetic acid; piperidine / toluene / Heating 4: sodium tetrahydroborate; butane-2,3-dione dioxime; cobalt(II) chloride hexahydrate / water; N,N-dimethyl-formamide / 4 h / 65 - 70 °C 5: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 5 steps 1: N,N-dimethyl-formamide / 14 h / 80 °C 2: triethylamine / dichloromethane / 4 h / 0 - 5 °C 3: acetic acid; piperidine / toluene / 4 h / 120 - 130 °C 4: sodium tetrahydroborate; butane-2,3-dione dioxime; cobalt(II) chloride hexahydrate / water; N,N-dimethyl-formamide / 3 h / 60 - 65 °C 5: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 5 steps 1: N,N-dimethyl-formamide / 14 h / 80 °C 2: triethylamine / dichloromethane / 4 h / 0 - 5 °C 3: acetic acid; piperidine / toluene / 4 h / 120 - 130 °C 4: sodium tetrahydroborate; butane-2,3-dione dioxime; cobalt(II) chloride hexahydrate / water; N,N-dimethyl-formamide / 3 h / 60 - 65 °C 5: hydrogenchloride / water; ethanol View Scheme |

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: acetic acid; piperidine / toluene / Heating 2.1: hydrogen / tetrahydrofuran / 12 h 3.1: zinc; acetic acid / 15 h / 25 - 30 °C 4.1: water; N-Bromosuccinimide / tert-butyl alcohol / 1.17 h / 25 - 30 °C 5.1: potassium carbonate / tert-butyl alcohol / 1.17 h / Reflux 5.2: 11 h / Reflux 6.1: pyrrolidine / methanol / 2 h / 50 - 55 °C / Large scale 7.1: sodium tetrahydroborate; butane-2,3-dione dioxime; cobalt(II) chloride hexahydrate / water; N,N-dimethyl-formamide / 4 h / 65 - 85 °C / Large scale 8.1: triethylamine 9.1: acetic acid; sodium tetrahydroborate / 15 h / 40 - 45 °C 10.1: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 10 steps 1.1: acetic acid; piperidine / toluene / Heating 2.1: hydrogen / tetrahydrofuran / 12 h 3.1: zinc; acetic acid / 15 h / 25 - 30 °C 4.1: water; N-Bromosuccinimide / dimethyl sulfoxide / 0.5 h / -5 - 0 °C 5.1: potassium carbonate / tert-butyl alcohol / 1.17 h / Reflux 5.2: 11 h / Reflux 6.1: pyrrolidine / methanol / 2 h / 50 - 55 °C / Large scale 7.1: sodium tetrahydroborate; butane-2,3-dione dioxime; cobalt(II) chloride hexahydrate / water; N,N-dimethyl-formamide / 4 h / 65 - 85 °C / Large scale 8.1: triethylamine 9.1: acetic acid; sodium tetrahydroborate / 15 h / 40 - 45 °C 10.1: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 10 steps 1.1: acetic acid; piperidine / toluene / Heating 2.1: hydrogen / tetrahydrofuran / 12 h 3.1: zinc; acetic acid / 15 h / 25 - 30 °C 4.1: water; N-Bromosuccinimide / dimethyl sulfoxide / 0.5 h / -5 - 0 °C 5.1: sodium hydride / N,N-dimethyl-formamide; mineral oil / 0.08 h 5.2: 15 h / 25 - 90 °C 6.1: pyrrolidine / methanol / 2 h / 50 - 55 °C / Large scale 7.1: sodium tetrahydroborate; butane-2,3-dione dioxime; cobalt(II) chloride hexahydrate / water; N,N-dimethyl-formamide / 4 h / 65 - 85 °C / Large scale 8.1: triethylamine 9.1: acetic acid; sodium tetrahydroborate / 15 h / 40 - 45 °C 10.1: hydrogenchloride / water; ethanol View Scheme |

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: acetic acid; piperidine / toluene / 4 h / 120 - 130 °C 2: sodium tetrahydroborate; butane-2,3-dione dioxime; cobalt(II) chloride hexahydrate / water; N,N-dimethyl-formamide / 3 h / 60 - 65 °C 3: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 4 steps 1: acetic acid; piperidine / toluene / 4 h / 120 - 130 °C 2: palladium 10% on activated carbon; hydrogen / methanol / 14 h / 3102.97 Torr 3: acetic acid; sodium tetrahydroborate / 15 h / 40 - 45 °C 4: hydrogenchloride / water; ethanol View Scheme |

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: acetic acid; piperidine / toluene / 4 h / 120 - 130 °C 2: sodium tetrahydroborate; butane-2,3-dione dioxime; cobalt(II) chloride hexahydrate / water; N,N-dimethyl-formamide / 3 h / 60 - 65 °C 3: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 3 steps 1: acetic acid; piperidine / toluene / 4 h / 120 - 130 °C 2: palladium 10% on activated carbon; hydrogen / water; methanol / 14 h / 3102.97 Torr 3: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 4 steps 1: acetic acid; piperidine / toluene / 4 h / 120 - 130 °C 2: palladium 10% on activated carbon; hydrogen / water; methanol / 14 h / 3102.97 Torr 3: acetic acid; sodium tetrahydroborate / 15 h / 40 - 45 °C 4: hydrogenchloride / water; ethanol View Scheme |
-
-
646519-89-3
5-{4-[2-chloro-2-(5-ethylpyridin-2-yl)ethoxy]benzyl}-2,4-thiazolidene dione hydrochloride

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: zinc; propionic acid / ethanol / 12 h 2: hydrogenchloride / water; ethanol View Scheme |
-
-
123-08-0
4-hydroxy-benzaldehyde

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: N-Bromosuccinimide / tert-butyl alcohol; water / 1.5 h / 25 °C / Large scale 1.2: 0.75 h / Large scale 1.3: PEG 4000 / 17 h / 78 °C / Large scale 2.1: thionyl chloride / chloroform / 1 h / Reflux 3.1: acetic acid; piperidine / toluene / Heating 4.1: hydrogen / tetrahydrofuran / 12 h 5.1: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 5 steps 1.1: N-Bromosuccinimide / tert-butyl alcohol; water / 1.5 h / 25 °C / Large scale 1.2: 0.75 h / Large scale 1.3: PEG 4000 / 17 h / 78 °C / Large scale 2.1: pyrrolidine / methanol / 2 h / 50 - 55 °C / Large scale 3.1: triethylamine / dichloromethane / 2 h / 0 °C 4.1: palladium 10% on activated carbon; hydrogen / water; methanol / 14 h / 3102.97 Torr 5.1: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 5 steps 1.1: sodium hydride / N,N-dimethyl-formamide; mineral oil / 0.08 h 1.2: 15 h / 25 - 90 °C 2.1: thionyl chloride / chloroform / 1 h / Reflux 3.1: acetic acid; piperidine / toluene / Heating 4.1: hydrogen / tetrahydrofuran / 12 h 5.1: hydrogenchloride / water; ethanol View Scheme |
-
-
471295-98-4
4-[2-(5-ethylpyridin-2-yl)-2-hydroxyethoxy]benzaldehyde

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: thionyl chloride / chloroform / 1 h / Reflux 2: acetic acid; piperidine / toluene / Heating 3: sodium tetrahydroborate; butane-2,3-dione dioxime; cobalt(II) chloride hexahydrate / water; N,N-dimethyl-formamide / 4 h / 65 - 70 °C 4: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 4 steps 1: thionyl chloride / chloroform / 1 h / Reflux 2: acetic acid; piperidine / toluene / Heating 3: hydrogen / tetrahydrofuran / 12 h 4: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 4 steps 1: triethylamine / dichloromethane / 4 h / 0 - 5 °C 2: acetic acid; piperidine / toluene / 4 h / 120 - 130 °C 3: sodium tetrahydroborate; butane-2,3-dione dioxime; cobalt(II) chloride hexahydrate / water; N,N-dimethyl-formamide / 3 h / 60 - 65 °C 4: hydrogenchloride / water; ethanol View Scheme |

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: sodium hydroxide / water / 2 h / 40 °C 2: thionyl chloride; triethylamine / dichloromethane / 1.25 h / 0 - 5 °C 3: N,N-dimethyl-formamide / 14 h / 80 °C 4: thionyl chloride / chloroform / 1 h / Reflux 5: acetic acid; piperidine / toluene / Heating 6: sodium tetrahydroborate; butane-2,3-dione dioxime; cobalt(II) chloride hexahydrate / water; N,N-dimethyl-formamide / 4 h / 65 - 70 °C 7: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 7 steps 1: sodium hydroxide / water / 2 h / 40 °C 2: thionyl chloride; triethylamine / dichloromethane / 1.25 h / 0 - 5 °C 3: N,N-dimethyl-formamide / 14 h / 80 °C 4: pyrrolidine / methanol / 2 h / 50 - 55 °C / Large scale 5: thionyl chloride / chloroform / 3 h / Reflux 6: sodium tetrahydroborate; butane-2,3-dione dioxime; cobalt(II) chloride hexahydrate / water; N,N-dimethyl-formamide / 4 h / 65 - 70 °C 7: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 7 steps 1: sodium hydroxide / water / 2 h / 40 °C 2: thionyl chloride; triethylamine / dichloromethane / 1.25 h / 0 - 5 °C 3: N,N-dimethyl-formamide / 14 h / 80 °C 4: pyrrolidine / methanol / 2 h / 50 - 55 °C / Large scale 5: triethylamine / dichloromethane / 2 h / 0 °C 6: palladium 10% on activated carbon; hydrogen / water; methanol / 14 h / 3102.97 Torr 7: hydrogenchloride / water; ethanol View Scheme |
-
-
5408-74-2
5-ethyl-2-vinyl-pyridine

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: N-Bromosuccinimide / tert-butyl alcohol; water / 1.5 h / 25 °C / Large scale 1.2: 0.75 h / Large scale 1.3: PEG 4000 / 17 h / 78 °C / Large scale 2.1: thionyl chloride / chloroform / 1 h / Reflux 3.1: acetic acid; piperidine / toluene / Heating 4.1: hydrogen / tetrahydrofuran / 12 h 5.1: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 5 steps 1.1: N-Bromosuccinimide / tert-butyl alcohol; water / 1.5 h / 25 °C / Large scale 1.2: 0.75 h / Large scale 1.3: PEG 4000 / 17 h / 78 °C / Large scale 2.1: pyrrolidine / methanol / 2 h / 50 - 55 °C / Large scale 3.1: triethylamine / dichloromethane / 2 h / 0 °C 4.1: palladium 10% on activated carbon; hydrogen / water; methanol / 14 h / 3102.97 Torr 5.1: hydrogenchloride / water; ethanol View Scheme | |
| Multi-step reaction with 5 steps 1.1: N-Bromosuccinimide / tert-butyl alcohol; water / 1.5 h / 25 °C / Large scale 1.2: 0.75 h / Large scale 1.3: PEG 4000 / 17 h / 78 °C / Large scale 2.1: triethylamine / dichloromethane / 4 h / 0 - 5 °C 3.1: acetic acid; piperidine / toluene / 4 h / 120 - 130 °C 4.1: palladium 10% on activated carbon; hydrogen / water; methanol / 14 h / 3102.97 Torr 5.1: hydrogenchloride / water; ethanol View Scheme |
-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

-
-
1134163-24-8
rac-5-({p-[2-(5-ethyl-2-pyridyl)ethoxy]phenyl}methyl)-(5-2H)-1,3-thiazolidine-2,4-dione

| Conditions | Yield |
|---|---|
| With triethylamine In dimethylsulfoxide-d6; d(4)-methanol at 20℃; for 108h; | 95% |
| With dimethylsulfoxide-d6; d(4)-methanol; triethylamine at 20℃; for 108h; | 95% |
| With dimethylsulfoxide-d6; d(4)-methanol; triethylamine at 20℃; for 108h; | 95% |
-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

-
-
21085-72-3
1-bromo-2,3,4-tri-O-acetyl-α-D-glucuronic acid methyl ester

-
-
1296832-69-3
3-((2R,3R,4S,5S,6S)-3,4,5-triacetoxy-6-methoxycarbonyl-tetrahydropyran-2-yl)-5-{4-[2-(5-ethyl-2-pyridinyl)ethoxy]benzyl}-thiazolidine-2,4-dione

| Conditions | Yield |
|---|---|
| With caesium carbonate In acetonitrile at 70℃; for 3h; | 83% |
-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

-
-
657-24-9
dimethylbiguanide

| Conditions | Yield |
|---|---|
| In water | 64% |
-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

-
-
7646-85-7
zinc(II) chloride

| Conditions | Yield |
|---|---|
| With sodium hydroxide In N,N-dimethyl-formamide at 70℃; pH=6 - 6.5; | 51.08% |
-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

-
-
1296832-75-1
3-((2R,3R,4S,5S,6S)-6-carboxy-3,4,5-trihydroxy-tetrahydropyran-2-yl)-5-{4-[2-(5-ethyl-2-pyridinyl)ethoxy]benzyl}-thiazolidine-2,4-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: caesium carbonate / acetonitrile / 3 h / 70 °C 2: hydrogenchloride / 1,4-dioxane / 76 h / 20 °C 3: morpholine; tetrakis(triphenylphosphine) palladium(0) / dichloromethane / 4 h / 20 °C View Scheme |
-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

-
-
1296832-76-2
(2S,3S,4S,5R,6R)-6-(1-carboxy-2-{4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl}-ethylsulfanylcarbonylamino)-3,4,5-trihydroxy-tetrahydropyran-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: caesium carbonate / acetonitrile / 3 h / 70 °C 2: hydrogenchloride / 1,4-dioxane / 76 h / 20 °C 3: morpholine; tetrakis(triphenylphosphine) palladium(0) / dichloromethane / 4 h / 20 °C 4: water; acetonitrile 5: sodium acetate / water; acetonitrile / 296 h / 20 °C View Scheme |
-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

-
-
1296832-72-8
3-((2R,3R,4S,5S,6S)-6-allyloxycarbonyl-3,4,5-trihydroxy-tetrahydropyran-2-yl)-5-{4-[2-(5-ethyl-2-pyridinyl)ethoxy]benzyl}-thiazolidine-2,4-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: caesium carbonate / acetonitrile / 3 h / 70 °C 2: hydrogenchloride / 1,4-dioxane / 76 h / 20 °C View Scheme |
-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: caesium carbonate / acetonitrile / 3 h / 70 °C 2: hydrogenchloride / 1,4-dioxane / 76 h / 20 °C 3: morpholine; tetrakis(triphenylphosphine) palladium(0) / dichloromethane / 4 h / 20 °C 4: water; acetonitrile View Scheme |
-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: caesium carbonate / acetonitrile / 3 h / 70 °C 2: hydrogenchloride / 1,4-dioxane / 76 h / 20 °C 3: morpholine; tetrakis(triphenylphosphine) palladium(0) / dichloromethane / 4 h / 20 °C 4: water; acetonitrile 5: sodium acetate / water; acetonitrile / 296 h / 20 °C 6: water; acetonitrile View Scheme |
-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

-
-
1263978-84-2
(5R)-5-{4-[2-(5-ethylpyridin-2-yl)ethoxy]benzyl}-1,3-thiazolidine-2,4-dione L-tartrate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: water; methanol / 96 h / Inert atmosphere 2.1: tetrahydrofuran / 1.5 h / 20 - 35 °C / Inert atmosphere 2.2: 20 °C / Reflux; Inert atmosphere View Scheme |
-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

-
-
1263978-86-4
(5R)-5-{4-[2-(5-ethylpyridin-2-yl)ethoxy]benzyl}-1,3-thiazolidine-2,4-dione tosylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: water; methanol / 96 h / Inert atmosphere 2: isopropyl alcohol / 0.5 h / 20 - 40 °C / Inert atmosphere View Scheme |
-
-
87-69-4
L-Tartaric acid

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

-
A
-
1263978-84-2
(5R)-5-{4-[2-(5-ethylpyridin-2-yl)ethoxy]benzyl}-1,3-thiazolidine-2,4-dione L-tartrate

| Conditions | Yield |
|---|---|
| In methanol; water for 96h; Inert atmosphere; | A n/a B n/a |

-
-
32634-66-5
Di-p-toluoyl-L-tartaric acid

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride


| Conditions | Yield |
|---|---|
| In methanol; water for 96h; Inert atmosphere; | A n/a B n/a |

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

-
-
2743-38-6
O,O'-dibenzoyl-L-tartaric acid


| Conditions | Yield |
|---|---|
| In methanol; water for 96h; Product distribution / selectivity; Inert atmosphere; | A n/a B n/a |

-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

-
A
-
1229114-66-2
3-(4-(2-(5-ethylpyridine-2yl)ethoxy)phenyl)-2-mercaptopropanoic acid

-
B
-
1229114-67-3
2-(1-carboxy-2-{4-[2-(5-ethylpyridine-2yl)-ethoxy]phenyl}-ethyldisulfanyl)-3-{4-[2-(5-ethylpyridine-2yl)-ethoxy]phenyl}propanoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 80℃; for 24h; |
-
-
112529-15-4
5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione hydrochloride

-
-
145350-09-0
rac-2-(2-{4-[(2,4-dioxothiazolidin-5-yl)methyl]phenoxy}ethyl)-5-ethylpyridine-1-oxide

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide at 60 - 80℃; for 24h; |
Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View

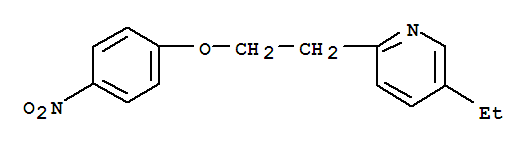







 Xi
Xi