Hebei yanxi chemical co.,LTD.
hebei yanxi chemical co., LTD who registered capital of 10 million yuan, nearly to $2 million, we have a pharmaceutical raw materials factory production of pharmaceutical raw materials, and a reagent r&d center, and we do research and developm
Cas:173334-57-1
Min.Order:1 Metric Ton
FOB Price: $1.0 / 3.0
Type:Manufacturers
inquiryWuhan Fortuna Chemical Co.,Ltd
Unique advantages for Aliskiren Cas 173334-57-1 Guaranteed purity High quality & competitive price Quality control Fast feedback Prompt shipment Appearance:Off-white to brown powder Storage:N/A Package:10g,100g,1kg/foil bag;25kg/drum
Cas:173334-57-1
Min.Order:10 Gram
FOB Price: $28.0 / 30.0
Type:Trading Company
inquiryHenan Allgreen Chemical Co.,Ltd
high quality Appearance:White or off-white Solid Storage:Sealed, dry, microtherm , avoid light and smell. Package:According to the demand of customer Application:Organic synthesis Transportation:by air or by sea Port:shanghai
Cas:173334-57-1
Min.Order:1 Kilogram
Negotiable
Type:Manufacturers
inquiryHangzhou JINLAN Pharm-Drugs Technology Co., Ltd
We can provide GMP validation service that complies with SFDA, FDA, WHO and EU EMPA.Excellent registration team could help us easlily to register our products in different countries.If you and your customer are interested in some products or need C
Cas:173334-57-1
Min.Order:1 Gram
Negotiable
Type:Manufacturers
inquiryXi'an Xszo Chem Co., Ltd.
1. Factory price and high quality must be guaranteed, base on 8 years of production and R&D experience2. Free samples will be provided,ensure specifications and quality are right for customer3. Customers will receive the most professional technical s
Cas:173334-57-1
Min.Order:1 Gram
FOB Price: $0.1
Type:Manufacturers
inquiryDayang Chem (Hangzhou) Co.,Ltd.
As a leading manufacturer and supplier of chemicals in China, DayangChem not only supply popular chemicals, but also DayangChem’s R&D center offer custom synthesis services. DayangChem can provide different quantities of custom synthesis
Chemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Henan Tianfu Chemical Co., Ltd.
TIANFUCHEM--173334-57-1--Aliskiren factory price Our company was built in 2009 with an ISO certificate.In the past 10 years, we have grown up as a famous fine chemicals supplier in China And we had established stable business relationsh
Cas:173334-57-1
Min.Order:1 Metric Ton
FOB Price: $2000.0
Type:Lab/Research institutions
inquiryLIDE PHARMACEUTICALS LIMITED
LIDE PHARMACEUTICALS LIMITED is a professional chemicals and APIs leading manufacturer in China. Our core business line covers APIs, Intermediates, Herb extract, etc.
Hebei Nengqian Chemical Import and Export Co., LTD
Our advantages: 1. All inquiries will be replied within 12 hours. 2. Dedication to quality, supply & service. 3. Strictly on selecting raw materials. 4. Reasonable & competitive price, fast lead time. 5. Sample is available for your eva
Zhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:173334-57-1
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryShanghai Upbio Tech Co.,Ltd
1.No Less 8 years exporting experience. Clients can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specialized
Cas:173334-57-1
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryQingdao Beluga Import and Export Co., LTD
Aliskiren CAS:173334-57-1 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organic intermediates,
Cas:173334-57-1
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Hebei Mojin Biotechnology Co.,Ltd
Company information: Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in chemical raw materials and chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big custo
Cas:173334-57-1
Min.Order:1 Kilogram
FOB Price: $8.0 / 10.0
Type:Trading Company
inquiryLonwin Chemical Group Limited
Aliskiren CAS: 173334-57-1 Specification Items Specification Result Assay ≥98% 99.25% Apperance Off-white to brown powder Off-white powder Loss on drying ≤0.5% 0.28%
Henan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Triumph International Development Limilted
Triumph has the complete production of G- KG - MT service chain,we can make the new technology into productivity quickly in the research and development of new products. Main Service 1.Own made fine chemical products 2.Out sourcin
Cas:173334-57-1
Min.Order:100 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryAfine Chemicals Limited
Our Services 1. New Molecules R&D 2. Own test center HPLC NMR GC LC-MS 3. API and Intermediates from China reputed manufacturers 4. Documents support COA MOA MSDS DMF open part Our advantages 1. Government awarded company. Top 100 enter
Wuhan Han Sheng New Material Technology Co.,Ltd
Our Advantage: high quality with competitive price High quality standard: BP/USP/EP Enterprise standard All purity customized Fast and safe delivery We have reliable forwarder who can help us deliver our goods more fast and safe. We
Cas:173334-57-1
Min.Order:10 Kilogram
Negotiable
Type:Trading Company
inquiryHangzhou Lingrui Chemical Co.,Ltd.
advantage: 1. The best price, satisfactory quality; 2. customers have the right to choose the delivery of parcels (EMS, DHL, FedEx, UPS); 3. customers have the right to choose from the recent effective packaging methods of their products packaging
Hangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Zibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:173334-57-1
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquirySHANGHAI SYSTEAM BIOCHEM CO., LTD
We are one of a few suppliers that can offer custom synthesis service of this product We are specialized in custom synthesis, chemical/pharmaceutical/ pesticides outsourcing and contract research. We are committed to prov
Cas:173334-57-1
Min.Order:100 Gram
FOB Price: $100.0 / 2000.0
Type:Lab/Research institutions
inquiryHANGZHOU YUNUO CHEMICAL CO.,LTD
Superior quality, moderate price & quick delivery. Appearance:white crystalline powder Storage:Stored in cool, dry and ventilation place; Away from fire and heat Package:100g/bottle,1kg/bottle,25kg/drum or as per your request Application:An
TaiChem Taizhou Limited
Established in May 2015, TaiChem Ltd. is initially invested by a British research and development company and started by PhDs back from aboard. The company is registered in China Medical City (CMC), Taizhou, Jiangsu Province, and the production site
Zibo Dorne chemical technology co. LTD
Product Details Grade: pharmaceutical grade Purity:99%+ ProductionCapacity: 1000 Kilogram/Month Scope of use: For scientific research only(The product must be used legally) Our Advantage 1. Best quality with competitive price. 2. Quick shipping,
Cas:173334-57-1
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryJiangsu Qianyu Molecular Technology Co., LTD.
Our Advantages A. International Top level TechnologyOur company owned biomedicine experts are famous at home and abroad with rich experience in research and development in the field of efficient chiral functional molecules research and development an
Xiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
EAST CHEMSOURCES LIMITED
factory?direct?saleAppearance:White Powder Storage:Store In Dry, Cool And Ventilated Place Package:25kg/drum, also according to the clients requirement Application:It is widely used as a thickener, emulsifier and stabilizer Transportation:By Sea Or B
Cas:173334-57-1
Min.Order:1 Kilogram
FOB Price: $18.0 / 20.0
Type:Trading Company
inquirySynthetic route
-
-
173338-07-3
tert-butyl (3S,5S,6S,8S)-8-(3-amino-2,2-dimethyl-3-oxopropylcarbamoyl)-6-hydroxy-3-(4-methoxy-3-(3-methoxypropoxy)benzyl)-2,9-dimethyldecan-5-ylcarbamate

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl (3S,5S,6S,8S)-8-(3-amino-2,2-dimethyl-3-oxopropylcarbamoyl)-6-hydroxy-3-(4-methoxy-3-(3-methoxypropoxy)benzyl)-2,9-dimethyldecan-5-ylcarbamate With hydrogenchloride In dichloromethane at 0 - 5℃; Stage #2: With sodium hydroxide In dichloromethane pH=9; | 100% |
| With hydrogenchloride In 1,4-dioxane at 0℃; Inert atmosphere; | 99% |
| With hydrogenchloride; water In ethyl acetate at 20℃; for 0.0833333h; Flow reactor; | 96% |
-
-
1236549-06-6
(1S,2S,4S)-4-(2-carbamoyl-2-methylpropyl-carbamoyl)-2-hydroxy-1-{(S)-2-[4-methoxy-3-(3-methoxypropoxy)-benzyl]-3-methylbutyl}-5-methylhexyl-carbamic acid benzyl ester

-
-
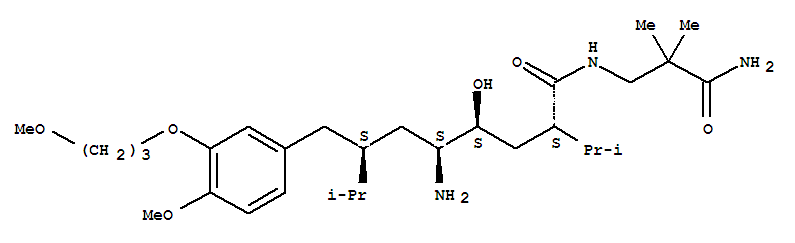
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With 10% palladium on activated charcoal; hydrogen | 97% |
| With 5%-palladium/activated carbon; hydrogen In tert-butyl methyl ether at 25℃; under 2280.15 Torr; for 16h; | 86% |
| With 5%-palladium/activated carbon; hydrogen In tert-butyl methyl ether at 25℃; under 2280.15 Torr; for 16h; | 86% |
| Stage #1: (1S,2S,4S)-4-(2-carbamoyl-2-methylpropyl-carbamoyl)-2-hydroxy-1-{(S)-2-[4-methoxy-3-(3-methoxypropoxy)-benzyl]-3-methylbutyl}-5-methylhexyl-carbamic acid benzyl ester With hydrogen; acetic acid; palladium 10% on activated carbon In ethanol at 20℃; under 760.051 Torr; Stage #2: With sodium hydroxide In ethanol pH=10; | |
| Stage #1: (1S,2S,4S)-4-(2-carbamoyl-2-methylpropyl-carbamoyl)-2-hydroxy-1-{(S)-2-[4-methoxy-3-(3-methoxypropoxy)-benzyl]-3-methylbutyl}-5-methylhexyl-carbamic acid benzyl ester With hydrogen; acetic acid; palladium 10% on activated carbon In ethanol at 20℃; under 760.051 Torr; Stage #2: With sodium hydroxide In ethanol; water pH=10; |

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With hydrogen; ethanolamine; 10% palladium on activated carbon In methanol at 20℃; under 760.051 Torr; for 3h; | 87% |
-
-
324763-51-1
aminopivalinamide

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With 2-hydroxypyridin; hydrogenchloride; triethylamine In methanol at 80℃; | 68% |


-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Stage #1: 3-amino-2,2-dimethylpropionamide ammonium chloride; 1,1-dimethylethyl[(1S,3S)-3-[{4-methoxy-3-(3-methoxypropoxy)phenyl}methyl]-4-methyl-1-[tetrahydro-4-(1-methylethyl)-5-oxo-2-furanyl]pentyl]carbamate; sodium 2-ethylhexanoic acid at 120℃; for 1h; Stage #2: With amine HCl Stage #3: With NaA Product distribution / selectivity; | 40% |

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen In methanol at 20℃; for 3h; | 38% |
-
-
324763-47-5
(2S,4S,5S,7S)-N-(3-amino-2,2-dimethyl-3-oxopropyl)-5-azido-4-hydroxy-2-isopropyl-7-(4-methoxy-3-(methoxypropoxy)benzyl)-8-methylnonanamide

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen; palladium on activated charcoal In methanol | |
| With 1% Pd/C; hydrogen In ethanol | |
| In methanol | |
| With hydrogen; ethanolamine; palladium 10% on activated carbon In isopropyl alcohol for 3h; Product distribution / selectivity; | |
| With palladium 10% on activated carbon; ammonia; hydrogen In ethanol at 20℃; under 5250.53 Torr; |
-
-
666844-61-7
(2-carbamoyl-2-methylpropyl)carbamic acid benzyl ester

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: H2 / Pd()OH)2/C 2: 65 percent / 2-hydroxypyridine; Et3N 3: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
172900-83-3
(1S,3S)-{1-formyl-3-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-4-methyl-pentyl}-carbamic acid tert-butyl ester

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: Mg 2: 23 percent / H2 / Pd(OH)2/C 3: 38 percent / TPAP; NMMO 4: 65 percent / 2-hydroxypyridine; Et3N 5: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
866030-33-3
(2R,5S)-3,6-diethoxy-2-isopropyl-5-((S)-2-(4-methoxy-3-(3-methoxypropoxy)benzyl)-3-methylbutyl)-2,5-dihydropyrazine

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: HCl / acetonitrile 2: Et3N 3: NaBH4 / ethanol 4: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 5: Mg 6: 23 percent / H2 / Pd(OH)2/C 7: 38 percent / TPAP; NMMO 8: 65 percent / 2-hydroxypyridine; Et3N 9: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
866030-35-5
tert-butyl {(1S,3S)-1-((2S,4S)-4-isopropyl-5-oxotetrahydrofuran-2-yl)-3-[4-methoxy-3-(3-methoxypropoxy)benzyl]-4-methylpentyl}carbamate

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 65 percent / 2-hydroxypyridine; Et3N 2: TMSCl; phenol / CH2Cl2 View Scheme | |
| Multi-step reaction with 2 steps 1.1: triethylamine; 2-hydroxypyridin / tert-butyl methyl ether / 18 h / 80 °C 2.1: trifluoroacetic acid / dichloromethane / 2 h / 20 °C 2.2: pH 10 View Scheme | |
| Multi-step reaction with 2 steps 1.1: triethylamine; 2-hydroxypyridin / 36 h / 60 - 65 °C 2.1: hydrogenchloride / dichloromethane; water / 3 h / 0 - 5 °C 2.2: 0 - 5 °C View Scheme |
-
-
866030-34-4
((1S,2S,4S)-2-hydroxy-4-hydroxymethyl-1-((S)-2-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-3-methyl-butyl)-5-methyl-hexyl)-carbamic acid tert-butyl ester

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 38 percent / TPAP; NMMO 2: 65 percent / 2-hydroxypyridine; Et3N 3: TMSCl; phenol / CH2Cl2 View Scheme |

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 23 percent / H2 / Pd(OH)2/C 2: 38 percent / TPAP; NMMO 3: 65 percent / 2-hydroxypyridine; Et3N 4: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
621-59-0
isovanillin

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 17 steps 1: 98 percent / K2CO3 / acetonitrile 2: NaBH4 / ethanol 3: PBr3 / CH2Cl2 4: 76 percent / LiHMDS / tetrahydrofuran 5: 81 percent / LiOH; H2O2 6: 95 percent / LAH / tetrahydrofuran 7: 97 percent / PPh3; NBS / CH2Cl2 8: 68 percent / n-BuLi 9: HCl / acetonitrile 10: Et3N 11: NaBH4 / ethanol 12: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 13: Mg 14: 23 percent / H2 / Pd(OH)2/C 15: 38 percent / TPAP; NMMO 16: 65 percent / 2-hydroxypyridine; Et3N 17: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
324763-51-1
aminopivalinamide

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 65 percent / 2-hydroxypyridine; Et3N 2: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
7505-93-3
2-cyano-2-methylpropanamide

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: LAH / tetrahydrofuran 1.2: Et3N 2.1: H2 / Pd()OH)2/C 3.1: 65 percent / 2-hydroxypyridine; Et3N 4.1: TMSCl; phenol / CH2Cl2 View Scheme | |
| Multi-step reaction with 3 steps 1: hydrogen; ammonia / raney nickel / methanol / 14 h / 40 - 45 °C / 2942.29 Torr 2: triethylamine; 2-hydroxypyridin / 16 h / 85 - 90 °C 3: hydrogen; ethanolamine / palladium 10% on activated carbon / isopropyl alcohol / 3 h View Scheme | |
| Multi-step reaction with 2 steps 1.1: hydrogen; ammonia / raney nickel / methanol / 14 h / 40 - 45 °C / 2942.29 Torr 2.1: 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone / 4 h / 75 - 80 °C 2.2: 15 h / 85 - 90 °C 2.3: 3 h View Scheme | |
| Multi-step reaction with 3 steps 1.1: ammonia / Raney nickel / methanol / 25 - 35 °C / Autoclave 1.2: 10 h / 60 - 65 °C / 5149.01 - 5884.58 Torr 2.1: triethylamine; 2-hydroxypyridin 3.1: hydrogenchloride / water; acetone / 20 °C 3.2: pH 8 - 9 View Scheme |
-
-
145589-03-3
(R)-4-benzyl-3-(3-methylbutyryl)oxazolidin-2-one

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1: 76 percent / LiHMDS / tetrahydrofuran 2: 81 percent / LiOH; H2O2 3: 95 percent / LAH / tetrahydrofuran 4: 97 percent / PPh3; NBS / CH2Cl2 5: 68 percent / n-BuLi 6: HCl / acetonitrile 7: Et3N 8: NaBH4 / ethanol 9: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 10: Mg 11: 23 percent / H2 / Pd(OH)2/C 12: 38 percent / TPAP; NMMO 13: 65 percent / 2-hydroxypyridine; Et3N 14: TMSCl; phenol / CH2Cl2 View Scheme | |
| Multi-step reaction with 9 steps 1: 50 percent / TiCl4 / CH2Cl2 2: 82 percent / LiOH; H2O2 3: 86 percent / LAH / tetrahydrofuran 4: 61 percent / PPh3; NBS / CH2Cl2 5: Mg 6: 23 percent / H2 / Pd(OH)2/C 7: 38 percent / TPAP; NMMO 8: 65 percent / 2-hydroxypyridine; Et3N 9: TMSCl; phenol / CH2Cl2 View Scheme | |
| Multi-step reaction with 13 steps 1.1: lithium hexamethyldisilazane / -78 °C / Inert atmosphere; Large scale 1.2: -78 °C / Inert atmosphere; Large scale 1.3: Inert atmosphere; Large scale 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 3 h / 0 - 20 °C / Inert atmosphere 3.1: triethylamine / toluene / 1.5 h / 0 - 20 °C / Inert atmosphere 4.1: sodium iodide / acetonitrile / 12 h / Inert atmosphere; Reflux 5.1: lithium hydride / N,N-dimethyl acetamide / 1 h / 60 °C / Inert atmosphere 5.2: 48 h / 60 °C / Inert atmosphere 6.1: dmap; triethylamine / 3 h / 20 °C / Inert atmosphere 7.1: trifluoroacetic acid / 1 h / 20 °C / Inert atmosphere 8.1: (R)-(-)-4,12-bis(diphenylphosphino)[2.2]paracyclophane(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; hydrogen; triethylamine / methanol / 24 h / 60 °C / 22502.3 Torr / Inert atmosphere; Large scale 9.1: diphenyl phosphoryl azide; triethylamine / toluene / 1 h / 80 °C / Inert atmosphere 9.2: 8 h / Inert atmosphere; Reflux 10.1: hydrogenchloride; water / ethanol / 48 h / 70 °C / Inert atmosphere 11.1: hydrogen; palladium 10% on activated carbon / methanol / 12 h / 20 °C / Inert atmosphere 11.2: 2 h / 20 °C / Inert atmosphere 12.1: 2-Ethylhexanoic acid / n-heptane / 8 h / 70 °C / Inert atmosphere 13.1: hydrogenchloride / dichloromethane / 3 h / -10 - 0 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 14 steps 1.1: diisopropylamine; n-butyllithium / tetrahydrofuran; hexane / 2 h / -78 °C / Inert atmosphere 1.2: 20 h / -78 - 20 °C 2.1: water; dihydrogen peroxide; lithium hydroxide / tetrahydrofuran / 3 h / 0 - 20 °C 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 6.5 h / 0 °C / Inert atmosphere; Reflux 4.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 1.5 h / 50 - 60 °C / Inert atmosphere 4.2: 20 °C 5.1: magnesium / tetrahydrofuran / 0.5 h / Inert atmosphere 5.2: -78 - 20 °C / Inert atmosphere 6.1: triethylamine; chlorotriethylstannane / 1,4-dioxane / 2 h / 0 °C 6.2: 20 °C 7.1: sodium periodate; ruthenium trichloride / acetonitrile / 0 - 20 °C 8.1: copper(II) sulfate / dichloromethane / 5 h / Reflux 9.1: boron trifluoride diethyl etherate / dichloromethane / 0.33 h / -78 - 20 °C 9.2: 5 h / 20 °C 10.1: diisobutylaluminium hydride / toluene / 2.5 h / -20 °C 11.1: magnesium / 0.5 h / Inert atmosphere 11.2: 3 h / -78 - 20 °C / Inert atmosphere 12.1: 10 wt% Pd(OH)2 on carbon; hydrogen / ethanol 13.1: 4-methylmorpholine N-oxide; tetrapropylammonium perruthennate / dichloromethane / 2 h / 20 °C / Inert atmosphere; Molecular sieve 14.1: triethylamine; 2-hydroxypyridin; hydrogenchloride / methanol / 80 °C View Scheme |
-
-
172900-75-3
4-methoxy-3-(3-methoxypropoxy)benzaldehyde

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 16 steps 1: NaBH4 / ethanol 2: PBr3 / CH2Cl2 3: 76 percent / LiHMDS / tetrahydrofuran 4: 81 percent / LiOH; H2O2 5: 95 percent / LAH / tetrahydrofuran 6: 97 percent / PPh3; NBS / CH2Cl2 7: 68 percent / n-BuLi 8: HCl / acetonitrile 9: Et3N 10: NaBH4 / ethanol 11: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 12: Mg 13: 23 percent / H2 / Pd(OH)2/C 14: 38 percent / TPAP; NMMO 15: 65 percent / 2-hydroxypyridine; Et3N 16: TMSCl; phenol / CH2Cl2 View Scheme | |
| Multi-step reaction with 13 steps 1.1: sodium tetrahydroborate / tetrahydrofuran / 0 - 30 °C 1.2: pH 2 2.1: phosphorus tribromide / dichloromethane / 2.17 h / 0 - 5 °C 3.1: lithium hexamethyldisilazane / tetrahydrofuran / 2 h / -70 °C / Inert atmosphere 3.2: -70 - 5 °C 4.1: dihydrogen peroxide; lithium hydroxide monohydrate / water; tetrahydrofuran / 0 - 30 °C 5.1: sodium tetrahydroborate / tetrahydrofuran / 1 h / 0 - 5 °C / Inert atmosphere 5.2: 5.75 h / 0 - 30 °C 6.1: N-chloro-succinimide; triphenylphosphine / dichloromethane / 5.5 h / -40 - 30 °C 7.1: magnesium; ethylene dibromide / tetrahydrofuran / 4.17 h / 63 - 68 °C / Inert atmosphere 7.2: 12.5 h / 0 - 5 °C 8.1: water; lithium hydroxide / tetrahydrofuran; methanol / 26 h / 65 - 70 °C 8.2: 0.5 h / 0 - 5 °C 9.1: hydrogenchloride / 1,1-dichloroethane; water / 0 - 5 °C / pH 2.5 9.2: 14.25 h / 0 - 30 °C 10.1: N-Bromosuccinimide; phosphoric acid / water; tetrahydrofuran / 1.25 h / 0 - 5 °C 11.1: sodium azide / ethylene glycol / 20 h / 80 - 85 °C 12.1: triethylamine; 2-hydroxypyridin / 16 h / 85 - 90 °C 13.1: hydrogen; ethanolamine / palladium 10% on activated carbon / isopropyl alcohol / 3 h View Scheme | |
| Multi-step reaction with 10 steps 1.1: sodium tetrahydroborate / tetrahydrofuran / 0 - 30 °C 1.2: pH 2 2.1: phosphorus tribromide / dichloromethane / 2.17 h / 0 - 5 °C 3.1: lithium hexamethyldisilazane / tetrahydrofuran / 2 h / -70 °C / Inert atmosphere 3.2: -70 - 5 °C 4.1: sodium tetrahydroborate / tetrahydrofuran / 0 - 15 °C 4.2: 11 h / 60 - 65 °C 5.1: N-chloro-succinimide; triphenylphosphine / dichloromethane / 5.5 h / -40 - 30 °C 6.1: magnesium; ethylene dibromide / tetrahydrofuran / 4.17 h / 63 - 68 °C / Inert atmosphere 6.2: 12.5 h / 0 - 5 °C 7.1: water; lithium hydroxide / tetrahydrofuran; methanol / 26 h / 65 - 70 °C 7.2: 0.5 h / 0 - 5 °C 8.1: hydrogenchloride / 1,1-dichloroethane; water / 0 - 5 °C / pH 2.5 8.2: 14.25 h / 0 - 30 °C 9.1: N-Bromosuccinimide; phosphoric acid / water; tetrahydrofuran / 1.25 h / 0 - 5 °C 10.1: 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone / 4 h / 75 - 80 °C 10.2: 15 h / 85 - 90 °C 10.3: 3 h View Scheme |
-
-
172900-70-8
(R)-2-[4-methoxy-3-(3-methoxypropoxy)benzyl]-3-methylbutanol

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1: 97 percent / PPh3; NBS / CH2Cl2 2: 68 percent / n-BuLi 3: HCl / acetonitrile 4: Et3N 5: NaBH4 / ethanol 6: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 7: Mg 8: 23 percent / H2 / Pd(OH)2/C 9: 38 percent / TPAP; NMMO 10: 65 percent / 2-hydroxypyridine; Et3N 11: TMSCl; phenol / CH2Cl2 View Scheme | |
| Multi-step reaction with 7 steps 1: 70 percent / SOCl2; pyridine 2: Mg; BrCH2CH2Br / tetrahydrofuran / 20 °C 3: H2 / [Ru2Cl4((S)-BINAP)]NEt3 / methanol / 40 h / 40 °C / 37503 Torr 4: NEt3 / CH2Cl2 5: 85 percent / NaN3; 15-crown-6; DMPU 6: 59 percent / 2-OH-pyridine; NEt3 7: H2; aq. HCl / Pd/C / methanol View Scheme | |
| Multi-step reaction with 8 steps 1.1: N-chloro-succinimide; triphenylphosphine / dichloromethane / 5.5 h / -40 - 30 °C 2.1: magnesium; ethylene dibromide / tetrahydrofuran / 4.17 h / 63 - 68 °C / Inert atmosphere 2.2: 12.5 h / 0 - 5 °C 3.1: water; lithium hydroxide / tetrahydrofuran; methanol / 26 h / 65 - 70 °C 3.2: 0.5 h / 0 - 5 °C 4.1: hydrogenchloride / 1,1-dichloroethane; water / 0 - 5 °C / pH 2.5 4.2: 14.25 h / 0 - 30 °C 5.1: N-Bromosuccinimide; phosphoric acid / water; tetrahydrofuran / 1.25 h / 0 - 5 °C 6.1: sodium azide / ethylene glycol / 20 h / 80 - 85 °C 7.1: triethylamine; 2-hydroxypyridin / 16 h / 85 - 90 °C 8.1: hydrogen; ethanolamine / palladium 10% on activated carbon / isopropyl alcohol / 3 h View Scheme |
-
-
172900-71-9
(R)-3-[4-methoxy-3-(3-methoxypropoxy)phenyl]-2-(1-methylethyl)propanoic acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1: 95 percent / LAH / tetrahydrofuran 2: 97 percent / PPh3; NBS / CH2Cl2 3: 68 percent / n-BuLi 4: HCl / acetonitrile 5: Et3N 6: NaBH4 / ethanol 7: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 8: Mg 9: 23 percent / H2 / Pd(OH)2/C 10: 38 percent / TPAP; NMMO 11: 65 percent / 2-hydroxypyridine; Et3N 12: TMSCl; phenol / CH2Cl2 View Scheme | |
| Multi-step reaction with 8 steps 1: 90 percent / NaBH4; I2 / 96 h / 20 °C 2: 70 percent / SOCl2; pyridine 3: Mg; BrCH2CH2Br / tetrahydrofuran / 20 °C 4: H2 / [Ru2Cl4((S)-BINAP)]NEt3 / methanol / 40 h / 40 °C / 37503 Torr 5: NEt3 / CH2Cl2 6: 85 percent / NaN3; 15-crown-6; DMPU 7: 59 percent / 2-OH-pyridine; NEt3 8: H2; aq. HCl / Pd/C / methanol View Scheme | |
| Multi-step reaction with 9 steps 1.1: sodium tetrahydroborate / tetrahydrofuran / 1 h / 0 - 5 °C / Inert atmosphere 1.2: 5.75 h / 0 - 30 °C 2.1: N-chloro-succinimide; triphenylphosphine / dichloromethane / 5.5 h / -40 - 30 °C 3.1: magnesium; ethylene dibromide / tetrahydrofuran / 4.17 h / 63 - 68 °C / Inert atmosphere 3.2: 12.5 h / 0 - 5 °C 4.1: water; lithium hydroxide / tetrahydrofuran; methanol / 26 h / 65 - 70 °C 4.2: 0.5 h / 0 - 5 °C 5.1: hydrogenchloride / 1,1-dichloroethane; water / 0 - 5 °C / pH 2.5 5.2: 14.25 h / 0 - 30 °C 6.1: N-Bromosuccinimide; phosphoric acid / water; tetrahydrofuran / 1.25 h / 0 - 5 °C 7.1: sodium azide / ethylene glycol / 20 h / 80 - 85 °C 8.1: triethylamine; 2-hydroxypyridin / 16 h / 85 - 90 °C 9.1: hydrogen; ethanolamine / palladium 10% on activated carbon / isopropyl alcohol / 3 h View Scheme |
-
-
365541-75-9
(2R)-3-methyl-2-[(phenylmethoxy)methyl]butan-1-ol

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 61 percent / PPh3; NBS / CH2Cl2 2: Mg 3: 23 percent / H2 / Pd(OH)2/C 4: 38 percent / TPAP; NMMO 5: 65 percent / 2-hydroxypyridine; Et3N 6: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
365541-74-8
(4R)-3-{(2S)-3-methyl-1-oxo-2-[(phenylmethoxy)methyl]butyl}-4-(phenylmethyl)oxazolidin-2-one

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 82 percent / LiOH; H2O2 2: 86 percent / LAH / tetrahydrofuran 3: 61 percent / PPh3; NBS / CH2Cl2 4: Mg 5: 23 percent / H2 / Pd(OH)2/C 6: 38 percent / TPAP; NMMO 7: 65 percent / 2-hydroxypyridine; Et3N 8: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
172901-00-7
(2S)-(benzyloxymethyl)-3-methyl-butyl bromide

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: Mg 2: 23 percent / H2 / Pd(OH)2/C 3: 38 percent / TPAP; NMMO 4: 65 percent / 2-hydroxypyridine; Et3N 5: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
656241-26-8
(2S)-3-methyl-2-[(phenylmethoxy)methyl]butanoic acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 86 percent / LAH / tetrahydrofuran 2: 61 percent / PPh3; NBS / CH2Cl2 3: Mg 4: 23 percent / H2 / Pd(OH)2/C 5: 38 percent / TPAP; NMMO 6: 65 percent / 2-hydroxypyridine; Et3N 7: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
172900-73-1
4-(bromomethyl)-1-methoxy-2-(3-methoxypropoxy)-benzene

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1: 76 percent / LiHMDS / tetrahydrofuran 2: 81 percent / LiOH; H2O2 3: 95 percent / LAH / tetrahydrofuran 4: 97 percent / PPh3; NBS / CH2Cl2 5: 68 percent / n-BuLi 6: HCl / acetonitrile 7: Et3N 8: NaBH4 / ethanol 9: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 10: Mg 11: 23 percent / H2 / Pd(OH)2/C 12: 38 percent / TPAP; NMMO 13: 65 percent / 2-hydroxypyridine; Et3N 14: TMSCl; phenol / CH2Cl2 View Scheme | |
| Multi-step reaction with 11 steps 1.1: lithium hexamethyldisilazane / tetrahydrofuran / 2 h / -70 °C / Inert atmosphere 1.2: -70 - 5 °C 2.1: dihydrogen peroxide; lithium hydroxide monohydrate / water; tetrahydrofuran / 0 - 30 °C 3.1: sodium tetrahydroborate / tetrahydrofuran / 1 h / 0 - 5 °C / Inert atmosphere 3.2: 5.75 h / 0 - 30 °C 4.1: N-chloro-succinimide; triphenylphosphine / dichloromethane / 5.5 h / -40 - 30 °C 5.1: magnesium; ethylene dibromide / tetrahydrofuran / 4.17 h / 63 - 68 °C / Inert atmosphere 5.2: 12.5 h / 0 - 5 °C 6.1: water; lithium hydroxide / tetrahydrofuran; methanol / 26 h / 65 - 70 °C 6.2: 0.5 h / 0 - 5 °C 7.1: hydrogenchloride / 1,1-dichloroethane; water / 0 - 5 °C / pH 2.5 7.2: 14.25 h / 0 - 30 °C 8.1: N-Bromosuccinimide; phosphoric acid / water; tetrahydrofuran / 1.25 h / 0 - 5 °C 9.1: sodium azide / ethylene glycol / 20 h / 80 - 85 °C 10.1: triethylamine; 2-hydroxypyridin / 16 h / 85 - 90 °C 11.1: hydrogen; ethanolamine / palladium 10% on activated carbon / isopropyl alcohol / 3 h View Scheme | |
| Multi-step reaction with 10 steps 1.1: lithium hexamethyldisilazane / tetrahydrofuran / 2 h / -70 °C / Inert atmosphere 1.2: -70 - 5 °C 2.1: sodium tetrahydroborate / tetrahydrofuran / 0 - 15 °C 2.2: 11 h / 60 - 65 °C 3.1: N-chloro-succinimide; triphenylphosphine / dichloromethane / 5.5 h / -40 - 30 °C 4.1: magnesium; ethylene dibromide / tetrahydrofuran / 4.17 h / 63 - 68 °C / Inert atmosphere 4.2: 12.5 h / 0 - 5 °C 5.1: water; lithium hydroxide / tetrahydrofuran; methanol / 26 h / 65 - 70 °C 5.2: 0.5 h / 0 - 5 °C 6.1: hydrogenchloride / 1,1-dichloroethane; water / 0 - 5 °C / pH 2.5 6.2: 14.25 h / 0 - 30 °C 7.1: N-Bromosuccinimide; phosphoric acid / water; tetrahydrofuran / 1.25 h / 0 - 5 °C 8.1: sodium azide / ethylene glycol / 20 h / 80 - 85 °C 9.1: triethylamine; 2-hydroxypyridin / 16 h / 85 - 90 °C 10.1: hydrogen; ethanolamine / palladium 10% on activated carbon / isopropyl alcohol / 3 h View Scheme |
-
-
172900-74-2
[4-methoxy-3-(3-methoxypropoxy)-phenyl]methanol

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 15 steps 1: PBr3 / CH2Cl2 2: 76 percent / LiHMDS / tetrahydrofuran 3: 81 percent / LiOH; H2O2 4: 95 percent / LAH / tetrahydrofuran 5: 97 percent / PPh3; NBS / CH2Cl2 6: 68 percent / n-BuLi 7: HCl / acetonitrile 8: Et3N 9: NaBH4 / ethanol 10: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 11: Mg 12: 23 percent / H2 / Pd(OH)2/C 13: 38 percent / TPAP; NMMO 14: 65 percent / 2-hydroxypyridine; Et3N 15: TMSCl; phenol / CH2Cl2 View Scheme | |
| Multi-step reaction with 12 steps 1.1: phosphorus tribromide / dichloromethane / 2.17 h / 0 - 5 °C 2.1: lithium hexamethyldisilazane / tetrahydrofuran / 2 h / -70 °C / Inert atmosphere 2.2: -70 - 5 °C 3.1: dihydrogen peroxide; lithium hydroxide monohydrate / water; tetrahydrofuran / 0 - 30 °C 4.1: sodium tetrahydroborate / tetrahydrofuran / 1 h / 0 - 5 °C / Inert atmosphere 4.2: 5.75 h / 0 - 30 °C 5.1: N-chloro-succinimide; triphenylphosphine / dichloromethane / 5.5 h / -40 - 30 °C 6.1: magnesium; ethylene dibromide / tetrahydrofuran / 4.17 h / 63 - 68 °C / Inert atmosphere 6.2: 12.5 h / 0 - 5 °C 7.1: water; lithium hydroxide / tetrahydrofuran; methanol / 26 h / 65 - 70 °C 7.2: 0.5 h / 0 - 5 °C 8.1: hydrogenchloride / 1,1-dichloroethane; water / 0 - 5 °C / pH 2.5 8.2: 14.25 h / 0 - 30 °C 9.1: N-Bromosuccinimide; phosphoric acid / water; tetrahydrofuran / 1.25 h / 0 - 5 °C 10.1: sodium azide / ethylene glycol / 20 h / 80 - 85 °C 11.1: triethylamine; 2-hydroxypyridin / 16 h / 85 - 90 °C 12.1: hydrogen; ethanolamine / palladium 10% on activated carbon / isopropyl alcohol / 3 h View Scheme | |
| Multi-step reaction with 10 steps 1.1: phosphorus tribromide / dichloromethane / 2.17 h / 0 - 5 °C 2.1: lithium hexamethyldisilazane / tetrahydrofuran / 2 h / -70 °C / Inert atmosphere 2.2: -70 - 5 °C 3.1: dihydrogen peroxide; lithium hydroxide monohydrate / water; tetrahydrofuran / 0 - 30 °C 4.1: sodium tetrahydroborate / tetrahydrofuran / 1 h / 0 - 5 °C / Inert atmosphere 4.2: 5.75 h / 0 - 30 °C 5.1: N-chloro-succinimide; triphenylphosphine / dichloromethane / 5.5 h / -40 - 30 °C 6.1: magnesium; ethylene dibromide / tetrahydrofuran / 4.17 h / 63 - 68 °C / Inert atmosphere 6.2: 12.5 h / 0 - 5 °C 7.1: water; lithium hydroxide / tetrahydrofuran; methanol / 26 h / 65 - 70 °C 7.2: 0.5 h / 0 - 5 °C 8.1: hydrogenchloride / 1,1-dichloroethane; water / 0 - 5 °C / pH 2.5 8.2: 14.25 h / 0 - 30 °C 9.1: N-Bromosuccinimide; phosphoric acid / water; tetrahydrofuran / 1.25 h / 0 - 5 °C 10.1: 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone / 4 h / 75 - 80 °C 10.2: 15 h / 85 - 90 °C 10.3: 3 h View Scheme |
-
-
172900-69-5
(R)-4-(2-(bromomethyl)-3-methylbutyl)-1-methoxy-2-(3-methoxypropoxy)benzene

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 68 percent / n-BuLi 2: HCl / acetonitrile 3: Et3N 4: NaBH4 / ethanol 5: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 6: Mg 7: 23 percent / H2 / Pd(OH)2/C 8: 38 percent / TPAP; NMMO 9: 65 percent / 2-hydroxypyridine; Et3N 10: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
172900-99-1
(2S,4S)-2-Amino-4-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-5-methyl-hexanoic acid ethyl ester

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: Et3N 2: NaBH4 / ethanol 3: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 4: Mg 5: 23 percent / H2 / Pd(OH)2/C 6: 38 percent / TPAP; NMMO 7: 65 percent / 2-hydroxypyridine; Et3N 8: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
172900-82-2
(2S,4S)-2-{[(tert-butoxy)carbonyl]amino}-4-[4-methoxy-3-(3-methoxypropoxy)benzyl]-5-methylhexan-1-ol

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 2: Mg 3: 23 percent / H2 / Pd(OH)2/C 4: 38 percent / TPAP; NMMO 5: 65 percent / 2-hydroxypyridine; Et3N 6: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
172900-76-4
ethyl (2S,4S)-2-{[(tert-butoxy)carbonyl]amino}-4-[4-methoxy-3-(3-methoxypropoxy)benzyl]-5-methylhexanoate

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: NaBH4 / ethanol 2: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 3: Mg 4: 23 percent / H2 / Pd(OH)2/C 5: 38 percent / TPAP; NMMO 6: 65 percent / 2-hydroxypyridine; Et3N 7: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
110-17-8
(2E)-but-2-enedioic acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| In ethanol; ethyl acetate | 93.6% |
| Stage #1: (2E)-but-2-enedioic acid; (2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide In ethanol; acetonitrile at 20 - 50℃; for 0.333333h; Stage #2: In ethanol; acetonitrile at 17 - 37℃; for 12.6667h; Product distribution / selectivity; aliskiren modification A; | |
| In methanol at 40 - 45℃; Temperature; Solvent; Concentration; | 3.8 g |
-
-
144-62-7
oxalic acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| In water for 0.333333h; | 93.36% |
-
-
110-15-6
succinic acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| In water for 0.25h; | 92.6% |
-
-
77-92-9
citric acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| In water for 0.5h; Product distribution / selectivity; | 92.37% |
-
-
110-17-8
(2E)-but-2-enedioic acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

-
-
173334-58-2
(2S,4S,5S,7S)-5-amino-N-(3-amino-2,2-dimethyl-3-oxypropyl)-4-hydroxy-7-[[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl]-8-methyl-(2-propan-2-yl)nonanamide hemifumarate

| Conditions | Yield |
|---|---|
| In methanol Solvent; Concentration; Time; Temperature; | 81.5% |
| In methanol at 35℃; for 1h; Product distribution / selectivity; | 74.25% |
| In ethanol at 20℃; Product distribution / selectivity; |
-
-
87-69-4
L-Tartaric acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| In ethanol; acetonitrile at 20℃; for 0.0833333h; Product distribution / selectivity; | 72.85% |
-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

-
-
1327153-75-2
(2S),(4S),(5S),(7S)-N-(3-amino-2,2-dimethyl-3-oxopropyl)-2,7-di(1-methylethyl)-4-hydroxy-5-amino-8-[4-methoxy-3-(3-methoxy-propoxy)phenyl]-octanamide phosphate

| Conditions | Yield |
|---|---|
| With phosphoric acid In water at 20℃; for 0.5h; Product distribution / selectivity; | 70.8% |
-
-
64-19-7
acetic acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

-
-
1327153-77-4
(2S),(4S),(5S),(7S)-N-(3-amino-2,2-dimethyl-3-oxopropyl)-2,7-di(1-methylethyl)-4-hydroxy-5-amino-8-[4-methoxy-3-(3-methoxy-propoxy)phenyl]-octanamide acetate

| Conditions | Yield |
|---|---|
| In water for 0.5h; | 65% |
-
-
97-67-6
(S)-Malic acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

-
-
1327153-72-9
(2S),(4S),(5S),(7S)-N-(3-amino-2,2-dimethyl-3-oxopropyl)-2,7-di(1-methylethyl)-4-hydroxy-5-amino-8-[4-methoxy-3-(3-methoxy-propoxy)phenyl]-octanamide L-malate

| Conditions | Yield |
|---|---|
| In water for 0.5h; Product distribution / selectivity; | 63.3% |
| In ethanol; dichloromethane at 20℃; for 24h; |
-
-
838878-70-9
pentafluorophenol 4-nitroxybutyrate

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In N,N-dimethyl-formamide at 0 - 20℃; for 4h; | 50% |
-
-
935472-60-9
4-(nitrooxy)butyl 4-nitrophenyl carbonate

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In N,N-dimethyl-formamide at 0 - 20℃; for 4h; | 50% |
-
-
838878-70-9
pentafluorophenol 4-nitroxybutyrate

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In N,N-dimethyl-formamide at 0 - 20℃; for 4h; | 45% |
-
-
874446-96-5
[4-(nitrooxy)methyl]benzoic acid pentafluorophenyl ester

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With dmap; scandium tris(trifluoromethanesulfonate) In N,N-dimethyl-formamide at 0 - 20℃; for 4h; | 30% |
-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With sulfuric acid In water; acetonitrile at 0 - 5℃; for 10 - 48h; | n/a |
| With sulfuric acid In water; acetonitrile at 0 - 5℃; for 11 - 49h; | n/a |
Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View