-
Name
(+)-Fenchol
- EINECS 216-639-5
- CAS No. 2217-02-9
- Article Data48
- CAS DataBase
- Density 0.992g/cm3
- Solubility Insoluble in water.
- Melting Point 39-45 ºC
- Formula C10H18 O
-
Boiling Point
201-202 °C(lit.)
- Molecular Weight 154.252
- Flash Point 165 ºF
- Transport Information
- Appearance
- Safety S22;S24/25
- Risk Codes 36/38
-
Molecular Structure
- Hazard Symbols
- Synonyms Bicyclo[2.2.1]heptan-2-ol,1,3,3-trimethyl-, (1R-endo)-; (+)-Fenchol; (+)-Fenchyl alcohol; (+)-a-Fenchol; (+)-a-Fenchyl alcohol;(1R)-endo-(+)-Fenchol; (1R)-endo-(+)-Fenchyl alcohol; (1R-endo)-Fenchol;D-Fenchol; D-Fenchyl alcohol; a-(+)-Fenchol; a-Fenchol, (+)-
- PSA 20.23000
- LogP 2.19350
Synthetic route

-
A
-
2217-02-9
(1R)-endo-(+)-fenchol

| Conditions | Yield |
|---|---|
| Stage #1: (1R)-1,3,3-trimethyl-2-(2-methylprop-2-enyl)bicyclo[2.2.1]heptan-2-ol With oxygen; ozone In diethyl ether at -78℃; Oxidation; Stage #2: With lithium aluminium tetrahydride In diethyl ether at -78 - 20℃; Reduction; | A 0.18 g B 79% |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; diethyl ether | |
| With ethanol; sodium | |
| With ammonia; lithium; ammonium chloride In tetrahydrofuran at -75℃; for 0.916667h; Yield given; |
-
-
10467-10-4
ethylmagnesium iodide

-
-
7787-20-4
(1R)-fenchone

-
A
-
2217-02-9
(1R)-endo-(+)-fenchol

-
B
-
137255-07-3
(1R)-endo-2-ethylfenchol

| Conditions | Yield |
|---|---|
| In diethyl ether for 8h; Heating; | A 84 % Chromat. B 13 % Chromat. |


| Conditions | Yield |
|---|---|
| With aluminum oxide; sodium; isopropyl alcohol In tetrahydrofuran for 5h; Heating; | A 44.0 % Chromat. B 56.0 % Chromat. |
| With aluminum isopropoxide; isopropyl alcohol for 420h; Heating; | A 1.02 g B n/a |
| With tropinone reductase of Cochlearia officinalis; NADPH In methanol at 30℃; for 1h; pH=5; Kinetics; Reagent/catalyst; Enzymatic reaction; |
-
-
146203-05-6
(+)-8-bromo-α-fenchol

-
-
2217-02-9
(1R)-endo-(+)-fenchol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether for 6h; Ambient temperature; |
-
-
251319-23-0
1-[(1R,2R)-2-hydroxy-1,3,3-trimethylbicyclo[2.2.1]hept-2-yl]propan-2-one

-
A
-
2217-02-9
(1R)-endo-(+)-fenchol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride Reduction; |
-
-
64-17-5
ethanol

-
-
7787-20-4
(1R)-fenchone

-
A
-
64439-31-2
(1R)-endo-(+)-fenchylalcohol

-
B
-
2217-02-9
(1R)-endo-(+)-fenchol


-
A
-
2217-02-9
(1R)-endo-(+)-fenchol

| Conditions | Yield |
|---|---|
| Stage #1: (1R)-2-ethenyl-1,3,3-trimethylbicyclo[2.2.1]heptan-2-ol With oxygen; ozone In diethyl ether at -78℃; Oxidation; Stage #2: With lithium aluminium tetrahydride In diethyl ether at -78 - 20℃; Reduction; | A 0.05 g B 0.07 g C n/a |


-
-
2217-02-9
(1R)-endo-(+)-fenchol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: O3/O2 / diethyl ether / -78 - 20 °C 2: LiAlH4 View Scheme | |
| Multi-step reaction with 2 steps 1.1: O3/O2 / diethyl ether / -78 °C 1.2: 8.28 g / Et3N / diethyl ether / -78 - 20 °C 2.1: LiAlH4 View Scheme |
-
-
18084-01-0, 21696-69-5, 131062-94-7, 137255-12-0, 137255-14-2
(1R,4S)-2-Ethynyl-1,3,3-trimethyl-bicyclo[2.2.1]heptan-2-ol

-
-
2217-02-9
(1R)-endo-(+)-fenchol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 59 percent Chromat. / N-methylpyrrolidone, potassium hydroxide / 4 h / 20 °C 2: 84 percent Chromat. / diethyl ether / 8 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| With octahydro-2,5-epiminopentalen-7-yloxidanyl; acetic acid; sodium nitrite In acetonitrile at 20℃; for 5h; air; | 100% |
| With Amberlyst A26 resin bound bis(azido)iodate(I) In acetonitrile at 20℃; for 6h; Irradiation; Inert atmosphere; | 99% |
| With 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In dimethyl sulfoxide for 0.25h; Ambient temperature; | 95% |
-
-
2217-02-9
(1R)-endo-(+)-fenchol

| Conditions | Yield |
|---|---|
| Stage #1: (1R)-endo-(+)-fenchol With n-butyllithium In tetrahydrofuran; diethyl ether at -30℃; for 0.166667h; Inert atmosphere; Schlenk technique; Stage #2: With trichlorophosphate In tetrahydrofuran; diethyl ether at -30 - 20℃; Inert atmosphere; Schlenk technique; | 99% |

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane for 0.2h; | 98% |
-
-
2217-02-9
(1R)-endo-(+)-fenchol

-
-
16000-39-8
1-cyano-2-methoxynaphthalene

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In 1,4-dioxane at 20℃; for 16h; Inert atmosphere; Sealed tube; Glovebox; | 98% |
-
-
2217-02-9
(1R)-endo-(+)-fenchol

| Conditions | Yield |
|---|---|
| With hypophosphorous acid In toluene; acetonitrile at 85℃; for 24h; | 97% |
-
-
916497-81-9
p-octyloxyphenyl 2-deoxy-2-phthalimido-3,4,6-tri-O-acetyl-1-thio-β-D-glucopyranoside

-
-
2217-02-9
(1R)-endo-(+)-fenchol

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; bis-[(trifluoroacetoxy)iodo]benzene In dichloromethane for 3h; Molecular sieve; | 97% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 36h; | 96% |
-
-
2217-02-9
(1R)-endo-(+)-fenchol

-
-
443988-47-4
benzyl 2-triethylsilyl-2-diazoacetate

| Conditions | Yield |
|---|---|
| dirhodium tetraacetate In toluene at 50℃; for 16h; | 94% |
-
-
2217-02-9
(1R)-endo-(+)-fenchol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
350849-01-3
endo-fenchyl tosylate

| Conditions | Yield |
|---|---|
| With pyridine at 60℃; for 168h; Inert atmosphere; | 94% |
-
-
2217-02-9
(1R)-endo-(+)-fenchol

-
-
2873-29-2
D-glucal triacetate

-
-
1170710-39-0
(1R)-endo-fenacholyl 4,6-di-O-acetyl-2,3-dideoxy-α-D-erythro-hex-2-enopyranoside

| Conditions | Yield |
|---|---|
| With gadolinium(III) trifluoromethanesulfonate In acetonitrile at 20 - 40℃; Catalytic behavior; Ferrier Carbohydrate Rearrangement; diastereoselective reaction; | 92% |
| With zinc dibromide In chloroform at 72℃; for 0.5h; Ferrier Carbohydrate Rearrangement; Microwave irradiation; stereoselective reaction; | 88% |
| With (1S)-10-camphorsulfonic acid In dichloromethane at 20℃; for 3h; Ferrier rearrangement; stereoselective reaction; | 84% |
| With triflic acid supported on silica gel at 40℃; for 0.166667h; Ferrier Carbohydrate Rearrangement; | 72% |
-
-
170097-87-7
6-(((tert-butoxycarbonyl)amino)methyl)nicotinic acid

-
-
2217-02-9
(1R)-endo-(+)-fenchol

| Conditions | Yield |
|---|---|
| Stage #1: 6-(((tert-butoxycarbonyl)amino)methyl)nicotinic acid With 2,4,6-trichlorobenzoyl chloride In N,N-dimethyl-formamide at 20℃; for 0.333333h; Stage #2: (1R)-endo-(+)-fenchol With dmap In N,N-dimethyl-formamide; toluene at 20℃; for 16h; | 90% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 5h; | 90% |
-
-
2217-02-9
(1R)-endo-(+)-fenchol

-
-
609-67-6
2-Iodobenzoyl chloride

-
-
1100747-72-5
(2R)-endo-fenchyl 2-iodobenzoate

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In acetonitrile at 80℃; | 88% |
-
-
2217-02-9
(1R)-endo-(+)-fenchol

-
-
26412-87-3
sulfur trioxide pyridine complex

| Conditions | Yield |
|---|---|
| With pyridine; acetic anhydride In toluene at 50℃; | 88% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 6h; | 88% |
-
-
2217-02-9
(1R)-endo-(+)-fenchol

-
-
108-24-7
acetic anhydride

-
-
99341-77-2
endo-bicyclo[2,2,1]heptan-2-ol-1,3,3-trimethyl acetate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; for 72h; Inert atmosphere; | 85% |
| With pyridine | |
| With sodium acetate |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene Dean-Stark; Reflux; | 85% |
| In toluene |
-
-
2217-02-9
(1R)-endo-(+)-fenchol

| Conditions | Yield |
|---|---|
| In dichloromethane for 16h; Ambient temperature; | 82% |

| Conditions | Yield |
|---|---|
| In toluene for 20h; Heating; | 79% |
-
-
2217-02-9
(1R)-endo-(+)-fenchol

| Conditions | Yield |
|---|---|
| In dichloromethane for 16h; Ambient temperature; | 78% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 12h; | 78% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 8h; | 78% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 10h; | 77% |

| Conditions | Yield |
|---|---|
| With [bis(trifluoromethanesulfonyl)imidate](triphenylphosphine)gold (I) at 150℃; for 0.5h; Inert atmosphere; Microwave irradiation; | 76% |

| Conditions | Yield |
|---|---|
| With lithium hexamethyldisilazane In tetrahydrofuran; hexane at -78℃; | 75% |
-
-
2217-02-9
(1R)-endo-(+)-fenchol

-
-
26607-51-2
N-benzyloxycarbonyl-D-alanine

-
-
108646-35-1
N-benzyloxycarbonyl-D-alanine (+)-α-fenchyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 6.5h; | 72.5% |

| Conditions | Yield |
|---|---|
| With 2-chloro-6-methylpyridinium triflate In hexane for 0.5h; Inert atmosphere; | 72% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 15 - 25℃; for 12h; Inert atmosphere; | 72% |
-
-
2217-02-9
(1R)-endo-(+)-fenchol

-
-
814-68-6
acryloyl chloride

-
-
219580-52-6
(1R,2R,4S)-1,3,3-trimethylbicyclo[2.2.1]heptan-2-yl acrylate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 1h; | 70% |
(+)-Fenchol Chemical Properties
Chemical Name: (+)-Fenchol
IUPAC NAME: (1S,4R,6S)-1,5,5-Trimethylbicyclo[2.2.1]heptan-6-ol
CAS No.: 2217-02-9
EINECS: 216-639-5
Molecular Formula: C10H18O
Molecular Weight: 154.25 g/mol
Melting Point: 43-46 °C
Density: 0.992 g/cm3
Flash Point: 73.9 °C
Boiling Point: 202.9 °C at 760 mmHg
Following is the structure of Fenchol (CAS No.:2217-02-9):
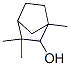
Product Categories about Fenchol (CAS No.:2217-02-9) is refer to Miscellaneous
The chemical synonymous of Fenchol (CAS No.:2217-02-9) are (+)-Fenchol ; Fenchol ; Fenchyl alcohol ; Fema 2480 ; Alpha Fenchol ; (1r)-1,3,3-Trimethylbicyclo[2.2.1]heptan-2-Ol ; (1r)-(+)-Fenchyl alcohol ; (1r)-Endo-(+)-fenchol
(+)-Fenchol Safety Profile
Risk Statements about Fenchol (CAS No.:2217-02-9):
R36/38:Irritating to eyes and skin.
Safety Statements about Fenchol (CAS No.:2217-02-9):
S22:Do not breathe dust.
S24/25:Avoid contact with skin and eyes.
Attention:
1. Storage: Store in a cool, dry place. Keep container closed when not in use.
2. Handling: Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View