-
Name
(1R,2R)-(-)-1,2-Diaminocyclohexane
- EINECS 211-776-7
- CAS No. 20439-47-8
- Article Data65
- CAS DataBase
- Density 0.939 g/cm3
- Solubility miscible
- Melting Point 41-45 °C
- Formula C6H14N2
- Boiling Point 193.6 °C at 760 mmHg
- Molecular Weight 114.191
- Flash Point 75 °C
- Transport Information UN 3259 8/PG 2
- Appearance white to light yellow crystal powder
- Safety 26-36/37/39-45
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms (1R,2R)-(-)-1,2-diaminocyclohexane acid;(1R,2R)-(-)-1,2-diamino cyclohexane;(1R, 2R)-2-Benzylamino-1-cyclopentanol;(R,R)-1,2-diaminocyclohexane;(1R,2R)-Cyclohexane-1,2-diamine;(1R,2R)-1,2-diaminocyclohexane;
- PSA 52.04000
- LogP 1.61560
Synthetic route

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water | 98% |
| With sodium hydroxide In dichloromethane; water at 20℃; for 0.5h; Inert atmosphere; | 96% |
| With potassium hydroxide In water | 92% |

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In ethanol at 20℃; under 1034.32 Torr; for 60h; | 97% |
-
-
329321-24-6
((1R,2R)-2-Azido-cyclohexyl)-((R)-1-phenyl-ethyl)-amine

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| With hydrogen; palladium dihydroxide In methanol at 20℃; under 2068.59 Torr; for 48h; | 95% |
-
-
147-71-7
D-tartaric acid

-
-
1121-22-8, 1436-59-5, 20439-47-8, 21436-03-3, 41013-43-8, 694-83-7
trans-1,2-Diaminocyclohexane

-
A
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In water at 70℃; Resolution of racemate; optical yield given as %ee; | A n/a B 95% |


-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water pH=9; | 92.9% |

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at -10 - -5℃; for 1h; | 92% |
-
-
1121-22-8, 1436-59-5, 20439-47-8, 21436-03-3, 41013-43-8, 694-83-7
trans-1,2-Diaminocyclohexane

-
-
108-59-8
malonic acid dimethyl ester

-
A
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

-
B
-
21436-03-3
(S,S)-1,2-diaminocyclohexane

-
C
-
186144-56-9
N-[(1R,2R)-2-(2-Methoxycarbonyl-acetylamino)-cyclohexyl]-malonamic acid methyl ester

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase on acrylic resin In 1,4-dioxane at 20℃; | A n/a B n/a C 48% D n/a |

-
-
1121-22-8, 1436-59-5, 20439-47-8, 21436-03-3, 41013-43-8, 694-83-7
trans-1,2-Diaminocyclohexane

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| With L-Tartaric acid |
-
-
694-83-7
1,2-diaminocyclohexane

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane
-
-
1121-22-8, 1436-59-5, 20439-47-8, 21436-03-3, 41013-43-8, 694-83-7
trans-1,2-Diaminocyclohexane

-
-
108-59-8
malonic acid dimethyl ester

-
A
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

-
B
-
21436-03-3
(S,S)-1,2-diaminocyclohexane

-
C
-
186144-56-9
N-[(1R,2R)-2-(2-Methoxycarbonyl-acetylamino)-cyclohexyl]-malonamic acid methyl ester

| Conditions | Yield |
|---|---|
| With immobilised Candida antarctica lipase (NOVOZYM 435) In 1,4-dioxane for 7h; |


-
A
-
57794-08-8
(1S,2S)-trans-1,2-Cyclohexanediol

-
B
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In benzene at 24.85℃; Equilibrium constant; Further Variations:; Solvents; dissociation; |

-
A
-
930-46-1
(1R,2R)-cyclopentane-1,2-diol

-
B
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In benzene at 24.85℃; Equilibrium constant; Further Variations:; Solvents; dissociation; |

-
A
-
63261-45-0
(S,S)-cyclopentane-1,2-diol

-
B
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In benzene at 24.85℃; Equilibrium constant; Further Variations:; Solvents; dissociation; |

-
A
-
1072-86-2, 1460-57-7, 1792-81-0, 54383-22-1, 57794-08-8, 931-17-9
(1R,2R)-trans-1,2-cyclohexanediol

-
B
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In benzene at 24.85℃; Equilibrium constant; Further Variations:; Solvents; dissociation; |
-
-
116407-32-0
(1R,2R)-(-)-1,2-diammoniumcyclohexane mono-L-(+)-tartrate

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol at 70 - 80℃; for 2h; | |
| With potassium carbonate In ethanol; water for 2h; Reflux; |
-
-
1121-22-8, 1436-59-5, 20439-47-8, 21436-03-3, 41013-43-8, 694-83-7
trans-1,2-Diaminocyclohexane

-
A
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

-
B
-
21436-03-3
(S,S)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| Stage #1: trans-1,2-Diaminocyclohexane With L-Tartaric acid; acetic acid In methanol; water for 24h; Heating; Stage #2: With hydrogenchloride In acetone for 25h; Heating; Stage #3: With sodium hydroxide | |
| Resolution of racemate; | |
| Stage #1: trans-1,2-Diaminocyclohexane With L-Tartaric acid In water at 70℃; for 2.5h; Resolution of racemate; Stage #2: With acetic acid In water for 2h; | |
| With C36H36N2O10 In methanol; diethyl ether at 10℃; for 0.5h; pH=4.5; Concentration; Solvent; Temperature; Time; pH-value; Resolution of racemate; enantioselective reaction; |
-
-
694-83-7
1,2-diaminocyclohexane

-
A
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

-
B
-
21436-03-3
(S,S)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| With (1S,4aS,10aR)-7-isopropyl-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydrophenanthrene-1-carboxylic acid In methanol |
-
-
286-20-4
cyclohexane-1,2-epoxide

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 95 percent / LiClO4 / acetonitrile / 48 h / 20 °C 2: 75 percent / diisopropyl azodicarboxylate; Ph3P / CH2Cl2 / 7 h / 0 - 20 °C 3: TMSN3 / acetonitrile / 7 h / 20 °C 4: 95 percent / H2 / Pd(OH)2/C / methanol / 48 h / 20 °C / 2068.59 Torr View Scheme |
-
-
329321-23-5
7-((R)-1-Phenyl-ethyl)-7-aza-bicyclo[4.1.0]heptane

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: TMSN3 / acetonitrile / 7 h / 20 °C 2: 95 percent / H2 / Pd(OH)2/C / methanol / 48 h / 20 °C / 2068.59 Torr View Scheme |
-
-
329321-22-4
2-((R)-1-Phenyl-ethylamino)-cyclohexanol

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 75 percent / diisopropyl azodicarboxylate; Ph3P / CH2Cl2 / 7 h / 0 - 20 °C 2: TMSN3 / acetonitrile / 7 h / 20 °C 3: 95 percent / H2 / Pd(OH)2/C / methanol / 48 h / 20 °C / 2068.59 Torr View Scheme |
-
-
492-99-9
1,2-cyclohexanedionedioxime

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: ethanol; sodium 2: Lg-tartaric acid View Scheme |

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: H2 / Pd-C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: (i) Fe2(SO4)3, NaN3, MeOH, (ii) H2O2, MeOH 2: H2 / Pd-C View Scheme |
-
-
694-83-7
1,2-diaminocyclohexane

-
A
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

-
B
-
1436-59-5
cis-cyclohexane-1,2-diamine

-
C
-
21436-03-3
(S,S)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| separation by salt formation with (+)-L-tartaric acid; | A 2.0 g B n/a C 0.9 g |


-
A
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

-
B
-
21436-03-3
(S,S)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| Stage #1: With titanium(IV) isopropylate; (R,R)-TADDOL In diphenylether at 90℃; for 1h; Stage #2: trans-1,2-cyclohexanediamine In diphenylether at 90℃; for 0.166667h; Product distribution / selectivity; | A n/a B n/a |
-
-
10027-80-2, 10027-83-5, 32044-18-1, 32044-21-6, 35018-62-3, 35018-63-4, 73571-37-6, 78020-70-9, 78995-00-3
(1R,2R)-1,2-dicyclohexane-1,2-diamine dihydrochloride

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| With sodium hydroxide |

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| With triethylamine; sodium hydroxide In dichloromethane at 0℃; for 0.166667h; |

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dichloromethane at 20℃; |

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| With potassium carbonate In water at 20℃; |
-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

-
-
100-52-7
benzaldehyde

-
-
199180-98-8
(1R,2R)-N,N'-dibenzylidene-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| 100% | |
| With 3 A molecular sieve In dichloromethane for 12h; | 91% |
| 90% |
-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

-
-
108-24-7
acetic anhydride

-
-
156257-14-6
trans-Diacetamido-(1R,2R)-(-)-1,2-cyclohexane

| Conditions | Yield |
|---|---|
| In chloroform for 1h; | 100% |
| With In(OSO2CF3)3 In acetonitrile at 20℃; Acetylation; | 91% |
| In methanol for 4h; Ambient temperature; | 73% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 24h; | 100% |
-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

-
-
100-52-7
benzaldehyde

-
-
65838-10-0, 135613-88-6, 143443-23-6
(1R,2R)-N,N'-dibenzylcyclohexane-1,2-diamine

| Conditions | Yield |
|---|---|
| Stage #1: (1R,2R)-1,2-diaminocyclohexane; benzaldehyde In methanol for 0.5h; Heating; Stage #2: With sodium tetrahydroborate In methanol for 0.25h; Heating; | 100% |
| Stage #1: (1R,2R)-1,2-diaminocyclohexane; benzaldehyde Stage #2: With sodium tetrahydroborate In methanol | 98% |
| Stage #1: (1R,2R)-1,2-diaminocyclohexane; benzaldehyde In methanol at 70℃; for 6h; Stage #2: With sodium tetrahydroborate In methanol at 20 - 70℃; for 4h; Stage #3: With hydrogenchloride; sodium hydroxide; water more than 3 stages; | 93% |
-
-
37942-07-7
3,5-di-tert-butyl-2-hydroxybenzaldehyde

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

-
-
135616-40-9
(R,R)-N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine

| Conditions | Yield |
|---|---|
| In ethanol for 1h; Heating; | 100% |
| With formic acid In methanol at 20℃; | 88% |
-
-
5431-44-7
2,6-Pyridinedicarboxaldehyde

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-Pyridinedicarboxaldehyde; (1R,2R)-1,2-diaminocyclohexane With barium(II) chloride In methanol for 25h; Stage #2: With sodium tetrahydroborate In methanol for 7h; Stage #3: With hydrogenchloride In methanol at 0℃; Further stages.; | 100% |
-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

-
-
616873-78-0
p-phenylenedioxydiacetic acid di-(3-tert-butyl-5-formyl-4-hydroxy-phenyl)ester

-
-
616873-79-1
1,3,5-benzenetrioxytriacetic acid tri-(3-tert-butyl-5-formyl-4-hydroxy-phenyl)ester

- polymer, triester/diester w/w feed ratio 0.5/100; monomer(s): 1,3,5-benzenetrioxytriacetic acid tri-(3-tert-butyl-5-formyl-4-hydroxyphenyl)ester; p-phenylenedioxydiacetic acid di-(3-tert-butyl-5-formyl-4-hydroxyphenyl)ester; (R,R)-1,2-diaminocyclohexane
-
polymer, triester/diester w/w feed ratio 0.5/100; monomer(s): 1,3,5-benzenetrioxytriacetic acid tri-(3-tert-butyl-5-formyl-4-hydroxyphenyl)ester; p-phenylenedioxydiacetic acid di-(3-tert-butyl-5-formyl-4-hydroxyphenyl)ester; (R,R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 2h; Heating; | 100% |

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

-
-
805314-84-5
(4-methoxy-phenoxy)-acetic acid 3-tert-butyl-5-formyl-4-hydroxy-phenyl ester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 2h; Heating; | 100% |
-
-
478282-20-1
5-tert-butyl-3-[(N,N-dimethylamino)methyl]-2-hydroxybenzaldehyde

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 24h; | 100% |
| In ethanol at 20℃; for 24h; |

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 24h; | 100% |
| In ethanol at 20℃; for 24h; |

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 6h; | 100% |
| In ethanol at 20℃; for 24h; | 100% |
-
-
478555-34-9
(R)-5-tert-butyl-2-hydroxy-3-{[methyl-(1-phenyl-ethyl)-amino]-methyl}-benzaldehyde

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 24h; | 100% |
| In ethanol at 20℃; for 24h; |
-
-
478555-35-0
(S)-5-tert-butyl-2-hydroxy-3-(2-methoxymethyl-pyrrolidin-1-ylmethyl)-benzaldehyde

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 24h; | 100% |
| In ethanol at 20℃; for 24h; |
-
-
336195-59-6
2,6-dichloro-heptanedioic acid bis-(3-tert-butyl-5-formyl-4-hydroxy-phenyl) ester

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 2h; | 100% |
-
-
98-03-3
thiophene-2-carbaldehyde

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

-
-
630402-57-2
(1R,2R)-N,N'-bis(thiophen-2-ylmethylene)cyclohexane-1,2-diamine

| Conditions | Yield |
|---|---|
| 100% | |
| With magnesium sulfate In chlorobenzene at 100℃; for 0.333333h; microwave irradiation; | 91% |
-
-
945629-73-2
4-[2,7-di-tert-butyl-5-(3-tert-butyl-5-formyl-4-hydroxyphenyl)-9,9-dimethyl-9H-xanthen-4-yl]benzoic acid methyl ester

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In ethanol for 12h; Heating; | 100% |
-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

-
-
70197-13-6
methyltrioxorhenium(VII)

| Conditions | Yield |
|---|---|
| In toluene equimolar organic ligand added to Re-compound in toluene at room temp.; filtrated; washed with n-hexane; elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 12h; | 100% |
| With trifluoroacetic acid In dichloromethane at 20℃; for 120h; | 83% |
| With trifluoroacetic acid In dichloromethane at 20℃; | 83% |
| With trifluoroacetic acid In dichloromethane at 20℃; | 83% |
| In dichloromethane at 20℃; | 70% |
-
-
1092119-19-1
3-tert-butyl-2-hydroxy-5-((6-oxo-1,6-dihydropyridin-2-yl)ethynyl)benzaldehyde

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 3h; Inert atmosphere; | 100% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In methanol | 100% |
-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

-
-
23165-29-9
3,5-bistrifluoromethylphenylisothiocyanate

| Conditions | Yield |
|---|---|
| In methanol for 0.333333h; | 100% |
-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

-
-
2131-61-5
p-nitrophenyl isothiocyanate

-
-
1402216-64-1
(1R,2R)-1,2-bis[N-(4-nitrophenyl)thiourea]cyclohexane

| Conditions | Yield |
|---|---|
| for 0.5h; neat (no solvent); | 100% |
-
-
1057558-12-9
1-(5-tert-butyl-3-formyl-2-hydroxybenzyl)pyridinium bromide

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 15h; Molecular sieve; | 100% |
-
-
1416171-26-0
3-(5-tert-butyl-3-formyl-2-hydroxybenzyl)-1-mesityl-1H-imidazol-3-ium chloride

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 20h; Inert atmosphere; Molecular sieve; | 100% |
-
-
1416171-27-1
3-(5-tert-butyl-3-formyl-2-hydroxybenzyl)-1-methyl-1H-benzo[d]imidazol-3-ium chloride

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 20h; Inert atmosphere; Molecular sieve; | 100% |
-
-
1416171-24-8
3-(5-(tert-butyl)-3-formyl-2-hydroxybenzyl)-1-methyl-1H-imidazol-3-ium chloride

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 20h; Inert atmosphere; Molecular sieve; | 100% |
-
-
1416171-25-9
3-(5-tert-butyl-3-formyl-2-hydroxybenzyl)-1-phenyl-1H-imidazol-3-ium chloride

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 20h; Inert atmosphere; Molecular sieve; | 100% |
-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

-
-
503065-08-5
2-(cyclohexylsulfanyl)benzaldehyde

-
-
1383611-02-6
(1R,2R)-N,N'-bis(2-(cyclohexylthio)benzylidene)-1,2-cyclohexanediamine

| Conditions | Yield |
|---|---|
| In ethanol at 80℃; | 100% |
-
-
53606-32-9
2-(isopropylsulfanyl)benzenecarbaldehyde

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

-
-
1383610-96-5
(1R,2R)-N,N'-bis(2-(isopropylthio)benzylidene)-1,2-cyclohexanediamine

| Conditions | Yield |
|---|---|
| In ethanol at 80℃; | 100% |
-
-
1383612-01-8
4-(tert-butyl)-2-(tert-butylthio)benzaldehyde

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

-
-
1383611-42-4
(1R,2R)-N,N-bis[4-(tert-butyl)-2-(tert-butylthio)benzylidene]-1,2-cyclohexanediamine

| Conditions | Yield |
|---|---|
| In ethanol at 80℃; | 100% |
(1R,2R)-(-)-1,2-Diaminocyclohexane Chemical Properties
IUPAC Name: (1R,2R)-Cyclohexane-1,2-diamine
Synonyms of (1R,2R)-(-)-1,2-Diaminocyclohexane (CAS NO.20439-47-8): (-)-R,R-1,2-Diaminocyclohexane ; (1R,2R)-1,2-Cyclohexanediamine ; (1R,2R)-Cyclohexan-1,2-diamin ;(1R,2R)-Cyclohexane-1,2-diamine ; (R,R)-DACH
Molecular Structure: 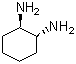
Molecular Formula: C6H14N2
Molecular Weight: 114.19
CAS NO: 20439-47-8
H bond acceptors: 2
H bond donors: 4
Freely Rotating Bonds: 2
Polar Surface Area: 6.48 Å2
Index of Refraction: 1.483
Molar Refractivity: 34.77 cm3
Molar Volume: 121.5 cm3
Surface Tension: 37 dyne/cm
Density: 0.939 g/cm3
Flash Point: 75 °C
Enthalpy of Vaporization: 42.98 kJ/mol
Boiling Point: 193.6 °C at 760 mmHg
Vapour Pressure: 0.46 mmHg at 25°C
Melting point: 41-45 °C
Storage temp: 2-8°C
Sensitive: Air Sensitive
Appearance: white to light yellow crystal powder
SMILES: N[C@@H]1CCCC[C@H]1N
InChI: InChI=1/C6H14N2/c7-5-3-1-2-4-6(5)8/h5-6H,1-4,7-8H2/t5-,6-/m1/s1
InChIKey: SSJXIUAHEKJCMH-PHDIDXHHBU
Std. InChI: InChI=1S/C6H14N2/c7-5-3-1-2-4-6(5)8/h5-6H,1-4,7-8H2/t5-,6-/m1/s1
Std. InChIKey: SSJXIUAHEKJCMH-PHDIDXHHSA-N
Product Categories of (1R,2R)-(-)-1,2-Diaminocyclohexane (CAS NO.20439-47-8): chiral;API intermediates;Chiral reagent;Amines (Chiral);Chiral Building Blocks;Synthetic Organic Chemistry;Chiral chemicals;Chiral Compound;Cycloalkanes
(1R,2R)-(-)-1,2-Diaminocyclohexane Uses
(1R,2R)-(-)-1,2-Diaminocyclohexane (CAS NO.20439-47-8) is used as Chiral synthesis of a variety of reagents, oxaliplatin intermediates and epoxy curing agent.
(1R,2R)-(-)-1,2-Diaminocyclohexane Production
Raw materials: Acetic acid glacial-->Potassium hydroxide -->tert-Butyl methyl ether-->L(+)-Tartaric acid-->D-Tartaric acid-->3-Methylbenzoyl chloride-->(+/-)-trans-1,2-Diaminocyclohexane
(1R,2R)-(-)-1,2-Diaminocyclohexane Safety Profile
Hazard Codes:  C
C
Risk Statements: 34
R34: Causes burns.
Safety Statements: 26-36/37/39-45
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection.
S45: In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
RIDADR: UN 3259 8/PG 2
WGK Germany: 3
F: 10-34
HazardClass: 8
PackingGroup: III
(1R,2R)-(-)-1,2-Diaminocyclohexane Specification
General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Combustible solid.
Extinguishing Media: Use water spray, dry chemical, carbon dioxide, or chemical foam.
Handling: Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Use only in a chemical fume hood.
Storage: Keep away from sources of ignition. Store in a cool, dry place. Store in a tightly closed container. Corrosives area. Store under nitrogen.
Related Products
- (1R)-(-)-(10-Camphorsulfonyl)oxaziridine
- (1R)-(-)-Menthyl acetate
- (1R)-(-)-Menthyl chloroformate
- (1R)-(-)-Menthyl glyoxylate hydrate
- (1R)-(-)-Thiocamphor
- (1R)-(+)-alpha-Pinene
- (1R)-1-(3-fluorophenyl)-2-methylpropylamine
- (1R)-1-[4-(benzyloxy)-3-nitrophenyl]-2-{[(2R)-1-(4-methoxyphenyl)propan-2-yl]amino}ethanol
- (1R)-3-[3-amino-4-(benzyloxy)phenyl]-2-{[(2R)-1-(4-methoxyphenyl)propan-2-yl]amino}ethan-1-ol
- (1R)-3-Chloro-1-phenyl-propan-1-ol
- 204395-49-3
- 20-44-0
- 204401-86-5
- 20440-91-9
- 20440-92-0
- 20440-93-1
- 20440-94-2
- 20440-95-3
- 20441-06-9
- 2044-21-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View