-
Name
(R)-1-PHENYL-2-PROPANOL
- EINECS
- CAS No. 1572-95-8
- Article Data127
- CAS DataBase
- Density 0.997 g/cm3
- Solubility
- Melting Point
- Formula C9H12O
- Boiling Point 219.999 °C at 760 mmHg
- Molecular Weight 136.194
- Flash Point 85 °C
- Transport Information
- Appearance
- Safety 23-24/25
-
Risk Codes
Xi:Irritant;
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms (R)-(-)-1-Phenyl-2-propanol;(-)-a-Methylphenethyl alcohol;(-)-a-Methylbenzeneethanol;(-)-1-Phenyl-2-propanol;Phenethylalcohol, a-methyl-, (-)- (8CI);Benzeneethanol,a-methyl-, (R)-;(R)-(-)-1-Phenyl-2-propanol;(R)-1-Phenyl-2-propanol;(R)-1-Phenyl-2-propanol;
- PSA 20.23000
- LogP 1.60990
Synthetic route

| Conditions | Yield |
|---|---|
| With magnesium chloride In isopropyl alcohol at 25℃; pH=7; Catalytic behavior; | 100% |
| With isopropyl alcohol; 1-butyl-3-methylimidazolium trifluoromethanesulfonimide at 40℃; pH=6; Microbiological reaction; | 95% |
| With ketoreductase P2-H07; isopropyl alcohol; NADPH In aq. phosphate buffer at 30℃; for 24h; pH=7; Reagent/catalyst; Enzymatic reaction; stereoselective reaction; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: bromobenzene With magnesium In tetrahydrofuran at 0℃; for 1h; Inert atmosphere; Stage #2: With copper(l) cyanide In tetrahydrofuran at -35℃; for 0.5h; Stage #3: (R)-propylene oxide In tetrahydrofuran at -35 - 20℃; for 1.5h; | 98% |
| With pyridine; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 4,4'-bipyridine; NiI2*3.9H2O; triethylamine hydrochloride; sodium iodide; zinc at 20℃; for 12h; | 86% |
-
-
14212-54-5
(+)-(R,R)-β-methylstyrene oxide

-
-
1572-95-8
(R)-1-phenyl-propan-2-ol

| Conditions | Yield |
|---|---|
| Stage #1: (+)-(R,R)-β-methylstyrene oxide With C30H32FeNPS at 20℃; for 5h; Stage #2: With hydrogenchloride In methanol for 1h; Reflux; | 93% |
| With hydrogen; Pd/magnetite In ethyl acetate at 23℃; under 760.051 Torr; for 0.7h; | |
| Multi-step reaction with 2 steps 1: aq. K2CO3 / Heating 2: (i) PBr5, CHCl3, (ii) Zn-Cu, aq. HCl View Scheme |

-
-
1572-95-8
(R)-1-phenyl-propan-2-ol

| Conditions | Yield |
|---|---|
| With methanol; potassium carbonate for 16h; Reflux; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| With triisopropoxytitanium(IV) chloride; (Ra)-2'-[(S)-hydroxy(pyridin-4-yl)methyl]-(1,1'-binaphthalen)-2-ol In diethyl ether; hexane at -20℃; for 0.166667h; enantioselective reaction; | 93% |

| Conditions | Yield |
|---|---|
| With air; cells of Geotrichum candidum IFO 5767 In water at 30℃; for 24h; | 80% |
-
-
132604-23-0
(R)-1-(2-Phenyl-[1,3]dithian-2-yl)-ethanol

-
-
1572-95-8
(R)-1-phenyl-propan-2-ol

| Conditions | Yield |
|---|---|
| With ethanol; nickel at 50℃; or nBu3SnH, AIBN, toluene, reflux; | 80% |
-
-
917-54-4
methyllithium

-
-
122-78-1
phenylacetaldehyde

-
A
-
1517-68-6
(S)-1-phenylpropan-2-ol

-
B
-
1572-95-8
(R)-1-phenyl-propan-2-ol

| Conditions | Yield |
|---|---|
| With triisopropoxytitanium(IV) chloride; (Sa)-2'-((S)-hydroxy(phenyl)methyl)-[1,1'-binaphthalen]-2-ol In diethyl ether; hexane at -20℃; for 0.166667h; enantioselective reaction; | A n/a B 80% |
| Stage #1: methyllithium; phenylacetaldehyde With (Sa)-2'-[(R)-hydroxy(phenyl)methyl]-(1,1'-binaphthalen)-2-ol; titanium(IV)isopropoxide In toluene at -40℃; for 1h; Inert atmosphere; Stage #2: With hydrogenchloride In water Inert atmosphere; optical yield given as %ee; enantioselective reaction; |


| Conditions | Yield |
|---|---|
| With methanesulfonic acid; (R,R)-dihydroborate In hexane at -20℃; for 48h; Title compound not separated from byproducts; | A n/a B 69% |
| With nicotinamide adenine dinucleotide; rat liver homogenate supernatant at 37℃; Product distribution; other catalysts; | |
| With lithium aluminium tetrahydride; crowned 2,2'-dihydroxy-1,1'-binaphthyl (S,S)-4 In tetrahydrofuran at 0℃; for 24h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |
-
-
698-87-3
3-phenyl-2-propanol

-
A
-
1517-68-6
(S)-1-phenylpropan-2-ol

-
B
-
1572-95-8
(R)-1-phenyl-propan-2-ol

-
C
-
103-79-7
1-phenyl-acetone

| Conditions | Yield |
|---|---|
| With (1R)-N-oxyl-1-(N-benzylcarbamoyl)-8-azabicyclo[3.2.1]octane; sodium hydrogencarbonate; sodium bromide In dichloromethane; water at 0℃; Electrochemical reaction; optical yield given as %ee; enantioselective reaction; | A n/a B n/a C 66% |
| With Sphingomonas paucimobilis NCIMB 8195 In water; N,N-dimethyl-formamide for 120h; | A n/a B n/a C 40% |
| With Geotrichum candidum IFO 4597 cells on BL-100 polymer; cyclohexanone In hexane at 30℃; for 24h; Oxidation; | A n/a B n/a C 44 % Chromat. |


| Conditions | Yield |
|---|---|
| With phosphate buffer; lipase at 20℃; | 47% |
| With sodium hydroxide; dm-3 phosphate buffer at 25℃; lipoprotein lipase from Pseudomonas aeruginosa (LPL Amano 1), pH 6.9-7.0; |

-
-
1572-95-8
(R)-1-phenyl-propan-2-ol

| Conditions | Yield |
|---|---|
| With phosphate buffer; lipase at 20℃; | 45% |
-
-
1254355-06-0
4-((1-methyl-2-phenylethoxy)carbonyl)butanoic acid

-
A
-
1414786-89-2
C14H18O4

-
C
-
1572-95-8
(R)-1-phenyl-propan-2-ol

| Conditions | Yield |
|---|---|
| With lipase from candida antartica In aq. phosphate buffer at 30℃; for 48h; | A n/a B n/a C 39% |

-
-
108-86-1
bromobenzene

-
-
15448-47-2
(R)-propylene oxide

-
A
-
37778-99-7
(S)-2-phenyl-1-propanol

-
B
-
1123-85-9, 37778-99-7, 98103-87-8, 19141-40-3
(+)-(R)-2-phenylpropanol

-
C
-
1572-95-8
(R)-1-phenyl-propan-2-ol

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; manganese; (1,2-dimethoxyethane)dichloronickel(II); bis(η5((1R,2S,5R)-5-methyl-2-[prop-2-yl]-cyclohex-1-yl)cyclopentadienyl)-titanium dichloride; sodium thiophenolate; triethylamine hydrochloride In benzene at 20℃; for 4h; Inert atmosphere; Glovebox; Autoclave; enantioselective reaction; | A n/a B n/a C 30% |

-
-
914929-68-3
3-((1-methyl-2-phenylethoxy)carbonyl)propanoic acid

-
A
-
1414786-86-9
C13H16O4

-
B
-
151585-66-9
C13H16O4

-
C
-
1572-95-8
(R)-1-phenyl-propan-2-ol

| Conditions | Yield |
|---|---|
| With lipase from candida antartica In aq. phosphate buffer at 30℃; for 48h; | A n/a B n/a C 22% |

-
-
108-86-1
bromobenzene

-
-
75-56-9, 16033-71-9
methyloxirane

-
A
-
37778-99-7
(S)-2-phenyl-1-propanol

-
B
-
1123-85-9, 37778-99-7, 98103-87-8, 19141-40-3
(+)-(R)-2-phenylpropanol

-
C
-
1572-95-8
(R)-1-phenyl-propan-2-ol

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; manganese; (1,2-dimethoxyethane)dichloronickel(II); bis(η5((1R,2S,5R)-5-methyl-2-[prop-2-yl]-cyclohex-1-yl)cyclopentadienyl)-titanium dichloride; triethylamine hydrochloride In benzene at 20℃; for 12h; Inert atmosphere; Glovebox; Autoclave; enantioselective reaction; | A n/a B n/a C 16% |

-
-
108-86-1
bromobenzene

-
-
75-56-9, 16033-71-9
methyloxirane

-
A
-
37778-99-7
(S)-2-phenyl-1-propanol

-
B
-
1517-68-6
(S)-1-phenylpropan-2-ol

-
C
-
1123-85-9, 37778-99-7, 98103-87-8, 19141-40-3
(+)-(R)-2-phenylpropanol

-
D
-
1572-95-8
(R)-1-phenyl-propan-2-ol

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; manganese; (1,2-dimethoxyethane)dichloronickel(II); bis(η5((1R,2S,5R)-5-methyl-2-[prop-2-yl]-cyclohex-1-yl)cyclopentadienyl)-titanium dichloride; triethylamine hydrochloride In benzene at 20℃; for 12h; Inert atmosphere; Glovebox; Autoclave; enantioselective reaction; | A n/a B n/a C 11% D 3% |

-
-
108-86-1
bromobenzene

-
-
75-56-9, 16033-71-9
methyloxirane

-
A
-
37778-99-7
(S)-2-phenyl-1-propanol

-
B
-
1123-85-9, 37778-99-7, 98103-87-8, 19141-40-3
(+)-(R)-2-phenylpropanol

-
C
-
1572-95-8
(R)-1-phenyl-propan-2-ol

-
D
-
66536-74-1
(S)-(+)-(2-hydroxy-1-propyl)-phenyl thioether

-
E
-
67253-47-8
(R)-2-hydroxypropane-1-phenylsulfide

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; manganese; (1,2-dimethoxyethane)dichloronickel(II); bis(η5((1R,2S,5R)-5-methyl-2-[prop-2-yl]-cyclohex-1-yl)cyclopentadienyl)-titanium dichloride; sodium thiophenolate; triethylamine hydrochloride at 20℃; for 2.5h; Inert atmosphere; Glovebox; Autoclave; enantioselective reaction; | A n/a B n/a C 9% D n/a E n/a |

-
-
698-87-3
3-phenyl-2-propanol

-
A
-
1517-68-6
(S)-1-phenylpropan-2-ol

-
B
-
2114-33-2, 29393-15-5, 115630-98-3, 116907-36-9
(R)-1-phenyl-2-acetoxypropane

-
C
-
1572-95-8
(R)-1-phenyl-propan-2-ol

| Conditions | Yield |
|---|---|
| Yield given. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
2114-33-2
1-phenylpropan-2-yl acetate

-
A
-
1517-68-6
(S)-1-phenylpropan-2-ol

-
B
-
2114-33-2, 29393-15-5, 115630-98-3, 116907-36-9
(R)-1-phenyl-2-acetoxypropane

-
C
-
1572-95-8
(R)-1-phenyl-propan-2-ol

-
D
-
29393-15-5
(S)-1-phenyl-propan-2-ol acetate

| Conditions | Yield |
|---|---|
| With water at 30℃; for 168h; Product distribution; asymmetric hydrolysis and resolution by Pseudomonas cepacia; | |
| With recombinant pig liver esterase In phosphate buffer for 2h; pH=7.5; Enzymatic reaction; |

-
-
2114-33-2, 29393-15-5, 115630-98-3, 116907-36-9
(R)-1-phenyl-2-acetoxypropane

-
-
1572-95-8
(R)-1-phenyl-propan-2-ol

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 25℃; for 4h; | |
| With lithium hydroxide In methanol at 20℃; for 3h; | |
| With sodium hydroxide In methanol; water | |
| With potassium hydroxide In tetrahydrofuran; methanol for 12h; | |
| With water; potassium hydroxide In methanol | n/a |
-
-
17596-79-1
(2S)-2-phenyl-1-propanamine

-
A
-
617-94-7
1-methyl-1-phenylethyl alcohol

-
B
-
93-54-9
1-Phenyl-1-propanol

-
C
-
1123-85-9
(RS)-2-phenyl-1-propanol

-
D
-
1517-68-6
(S)-1-phenylpropan-2-ol

-
E
-
1572-95-8
(R)-1-phenyl-propan-2-ol

| Conditions | Yield |
|---|---|
| With sodium nitrite In perchloric acid; water Product distribution; Mechanism; |

-
-
157752-41-5
rac-1-phenyl-2-propyl-propionate

-
A
-
1517-68-6
(S)-1-phenylpropan-2-ol

-
B
-
116809-20-2
propionic acid-((S)-1-methyl-2-phenyl-ethyl ester)

-
C
-
1572-95-8
(R)-1-phenyl-propan-2-ol

-
D
-
116809-20-2
Propionic acid (R)-1-methyl-2-phenyl-ethyl ester

| Conditions | Yield |
|---|---|
| With water at 30℃; for 168h; Product distribution; asymmetric hydrolysis and resolution by Pseudomonas cepacia; |

-
-
135559-47-6
(1R,2S,SR)-1-methyl-2-(methylsulfinyl)phenethyl alcohol

-
-
1572-95-8
(R)-1-phenyl-propan-2-ol

| Conditions | Yield |
|---|---|
| With nickel In tetrahydrofuran for 2h; Ambient temperature; |
-
-
116809-20-2
Propionic acid (R)-1-methyl-2-phenyl-ethyl ester

-
-
1572-95-8
(R)-1-phenyl-propan-2-ol

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 25℃; for 4h; | |
| With sodium hydroxide In methanol; water |
-
-
90472-61-0
((R)-1-Methyl-2-phenyl-ethoxy)-diphenyl-silane

-
-
1572-95-8
(R)-1-phenyl-propan-2-ol

| Conditions | Yield |
|---|---|
| With hydrogen cation In water Yield given; |

| Conditions | Yield |
|---|---|
| for 24h; Lactobacillus kefir; Yield given; |
-
-
40560-98-3
(1S,2R)-1-phenylpropane-1,2-diol

-
-
1572-95-8
(R)-1-phenyl-propan-2-ol

| Conditions | Yield |
|---|---|
| (i) PBr5, CHCl3, (ii) Zn-Cu, aq. HCl; Multistep reaction; |
-
-
698-87-3
3-phenyl-2-propanol

-
-
98-88-4
benzoyl chloride

-
A
-
1517-68-6
(S)-1-phenylpropan-2-ol

-
B
-
1572-95-8
(R)-1-phenyl-propan-2-ol

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve; chiral diamine; triethylamine In dichloromethane at -78℃; for 3h; Yield given; Yields of byproduct given; |

-
-
1572-95-8
(R)-1-phenyl-propan-2-ol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
61342-56-1
(R)-1-phenylpropan-2-yl 4-methylbenzenesulfonate

| Conditions | Yield |
|---|---|
| With trimethylamine hydrochloride; triethylamine In dichloromethane at 0℃; Inert atmosphere; | 99% |
| With pyridine In dichloromethane at 20℃; for 48h; | 90% |
| With pyridine; dmap at 30℃; for 3h; |
-
-
1572-95-8
(R)-1-phenyl-propan-2-ol

-
-
752242-26-5
methyl 3-hydroxy-5-isopropoxybenzoate

-
-
915948-80-0
methyl 3-isopropoxy-5-[(1S)-1-methyl-2-phenylethoxy]benzoate

| Conditions | Yield |
|---|---|
| Stage #1: (R)-1-phenyl-propan-2-ol; methyl 3-hydroxy-5-isopropoxybenzoate With triphenylphosphine In dichloromethane at 0℃; for 0.5h; Stage #2: With di-isopropyl azodicarboxylate In dichloromethane at 0 - 20℃; Stage #3: (R)-1-phenyl-propan-2-ol With di-isopropyl azodicarboxylate; triphenylphosphine at 0 - 20℃; for 2.83333h; | 97% |

| Conditions | Yield |
|---|---|
| With C10H10Zr(2+)*2CF3O3S(1-)*C4H8O at 80℃; for 24h; Sealed tube; | 93% |
-
-
1572-95-8
(R)-1-phenyl-propan-2-ol

-
-
124-63-0
methanesulfonyl chloride

-
-
1309251-32-8
(R)-1-phenylpropanyl-2-yl methansulfonate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 1h; | 92% |
| With pyridine at -15 - 20℃; for 2h; | 87% |
| With triethylamine In dichloromethane at 20℃; Inert atmosphere; |
-
-
1572-95-8
(R)-1-phenyl-propan-2-ol

-
-
61342-56-1
(R)-1-phenylpropan-2-yl 4-methylbenzenesulfonate

| Conditions | Yield |
|---|---|
| With pyridine; p-toluenesulfonyl chloride In dichloromethane at 20℃; for 100h; | 90% |
-
-
1572-95-8
(R)-1-phenyl-propan-2-ol

-
-
10304-81-1, 55449-46-2, 116698-42-1, 16583-73-6
(+)-2-chloro-1-phenylpropane

| Conditions | Yield |
|---|---|
| With pyridine; bis(trichloromethyl) carbonate In dichloromethane at 0℃; Reflux; | 89% |
-
-
863504-77-2
3-hydroxy-5-((S)-2-methoxy-1-methyl-ethoxy)-benzoic acid methyl ester

-
-
1572-95-8
(R)-1-phenyl-propan-2-ol

-
-
915948-88-8
3-((S)-2-methoxy-1-methyl-ethoxy)-5-((S)-1-methyl-2-phenyl-ethoxy)-benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 3-hydroxy-5-((S)-2-methoxy-1-methyl-ethoxy)-benzoic acid methyl ester; (R)-1-phenyl-propan-2-ol With triphenylphosphine In tetrahydrofuran at 0℃; for 0.0833333h; Stage #2: With di-isopropyl azodicarboxylate In tetrahydrofuran at 20℃; for 12h; | 81% |
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 0 - 20℃; for 12.0833h; | 81% |
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 0 - 20℃; for 16h; | 80% |
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran; toluene at 0 - 20℃; |

| Conditions | Yield |
|---|---|
| Stage #1: (R)-1-phenyl-propan-2-ol With n-butyllithium In tetrahydrofuran; hexane at -70℃; for 0.5h; Stage #2: bis(diethylamino)chlorophosphine In tetrahydrofuran; hexane at -70 - 20℃; | 80% |
-
-
1572-95-8
(R)-1-phenyl-propan-2-ol

-
-
2114-39-8, 14367-52-3, 63798-14-1, 130232-93-8
(S)-2-bromo-1-phenylpropane

| Conditions | Yield |
|---|---|
| With 1H-imidazole; bromine; triphenylphosphine In dichloromethane at 0 - 20℃; | 68% |
| With carbon tetrabromide; triphenylphosphine |
-
-
1572-95-8
(R)-1-phenyl-propan-2-ol

-
-
121-44-8
triethylamine

-
A
-
1388835-90-2
(-)-(R)-1-phenylpropan-2-yl diethylcarbamate

-
B
-
10304-81-1, 55449-46-2, 116698-42-1, 16583-73-6
(+)-2-chloro-1-phenylpropane

| Conditions | Yield |
|---|---|
| With bis(trichloromethyl) carbonate In dichloromethane at 0 - 20℃; Solvent; Concentration; | A 27% B 45% |

-
-
1122-28-7
4,5-dicyano-1H-imidazole

-
-
1572-95-8
(R)-1-phenyl-propan-2-ol

-
-
162989-77-7
(S)-(+)-1-(1-Phenyl-2-propyl)imidazole-4,5-dicarbonitrile

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran at 0℃; for 0.5h; | 39% |
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran for 0.5h; Ambient temperature; | 39% |
-
-
1572-95-8
(R)-1-phenyl-propan-2-ol

| Conditions | Yield |
|---|---|
| With triphenylphosphine, polymer bound In 1,2-dimethoxyethane at 20℃; | 13% |
(2R)-1-Phenyl-2-propanol Chemical Properties
Molecular Structure of (R)-1-Phenyl-2-propanol (1572-95-8):
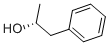
Systematic Name: (2S)-1-Phenylpropan-2-ol
Molecular Formula: C9H12O
Molecular Weight: 136.191
H bond acceptors: 1
H bond donors: 1
Freely Rotating Bonds: 3
Index of Refraction: 1.525
Molar Refractivity: 41.92 cm3
Molar Volume: 136.6 cm3
Surface Tension: 37.3 dyne/cm
Density: 0.996 g/cm3
Flash Point: 85 °C
Boiling Point: 220 °C at 760 mmHg
Enthalpy of Vaporization: 48.24 kJ/mol
Vapour Pressure: 0.067 mmHg at 25 °C
Water Solubility: 5838 mg/L at 25 °C
Refractive Index: n20/D 1.521
(2R)-1-Phenyl-2-propanol Safety Profile
Safety Information of (R)-1-Phenyl-2-propanol (1572-95-8):
Hazard Codes: Xi ![]()
Hazard Note: Irritant
Safety Statements: 23-24/25
23: Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer)
24/25: Avoid contact with skin and eyes
WGK Germany: 3
(2R)-1-Phenyl-2-propanol Specification
(R)-1-Phenyl-2-propanol (1572-95-8) is also known as R(-)-1-Phenyl-2-propanol ; R(-)-alpha-Methylphenethyl alcohol ; (2R)-1-Phenyl-2-propanol ; (R)-1-Phenylpropan-2-ol ; (R)-α-Methylbenzeneethanol . It is often used in organic synthesis.
Related Products
- (2R)-1-Phenyl-2-propanol
- 15729-58-5
- 157296-96-3
- 157296-98-5
- 1572-97-0
- 1572-99-2
- 15732-02-2
- 157327-41-8
- 157327-43-0
- 157327-44-1
- 157327-45-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View